
Green Chemistry in Teaching Laboratory
Microwave Induced Reactions
Protein Denaturization by Heat
Denaturation is the process of modifying the conformation of the protein structures without rupturing the native peptide linkages. This inactivates the functionality of the protein molecules, decreases its solubility, decreases/destroys its biological activity, improves digestibility and alters the water binding ability of the molecule. Denaturation of proteins is achieved by disrupting the hydrogen bonding in the peptide linkage by applying external stress. It can be carried out by applying heat, treatment with alcohols, heavy metals, or acids/bases.
The principle of denaturation has been exploited in the field of food chemistry and in developing genetically engineered compounds. The purpose of denaturing a protein is to inactivate certain components of a living cell, thereby suppressing it from expressing certain properties of the proteins. Protein denaturation is widely used in food processing and dairy industries and a simple example is the cooking of egg white for making an omelets.
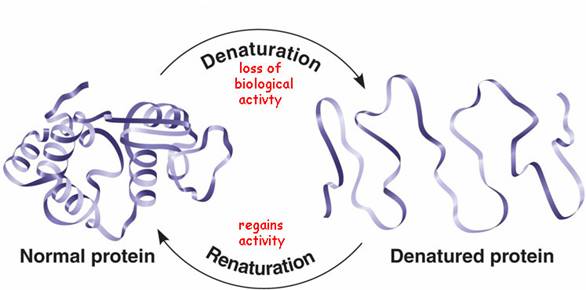
* Figure 6. A schematic representation for denaturation of proteins.
* Source: http://www.wikipedia.org
Materials Required:
-
Two 150ml calibrated glass beakers.
-
Two glass rods for stirring.
-
Two Eggs.
-
Safety goggles.
-
Towels for clean up.
-
Stop watch.
Experimental Procedure
About 100ml of egg white was separated by slightly cracking the freshly obtained eggs in two separate 150ml beakers. One beaker was placed in the microwave oven and the other on a hotplate. Both specimens were heated to a temperature of 100 o C. The applied heat caused the breakage of the long peptide bonds of the protein molecules which coagulates as white semi-solid structures. The time taken for coagulation by microwave oven and the hot plate were calculated to be 2 and 14 min respectively. The formation of coagulates are shown in the Figure 7 below.


(a) (b)
Figure 7. Photograph of egg white (a) separated from pure egg after coagulation and (b) before coagulation.
Result & Discussion:
As a result, the protein constituent separated from the egg on denaturation produced, white fluffy solids indicating the termination of the process. The energy consumption by each method are tabulated below.
Table 3: The energy consumed for the protein denaturation by different heating methods.
|
Heating Device |
Time (min) |
Power Rating (KJ/min) |
Actual Energy Consumed (KJ) |
|
Microwave Oven |
2 |
51.0 |
72 |
|
Hot Plate |
14 |
51.9 |
288 |
Therefore, the percentage of energy saved by the microwave oven over the conventional oven as recorded by the power meter is,
![]()
From the above experiment, the time taken for denaturation of protein by conventional heating was higher than microwave heating. This is due to the fundamental difference in the heating mechanisms. With microwave heating it was possible to locally heat the target molecules rather than maintaining a whole vessel at an elevated temperature. The former was a more energy-efficient process.