We already have been talking about
several forms of light--visible light, infrared, ultraviolet. In fact,
there are several more, and they are all exactly the same phenomenon--an electromagnetic
wave--and they all have the same speed (the speed of light)--but they differ
in wavelength (or equivalently, in frequency). Electromagnetic waves
are different from other waves in one important way: they do not need anything
to "wave" in! They can travel in a vacuum. Sound waves, for example,
cannot--they have to have something to travel through.
Here is an overview of the forms
of light:
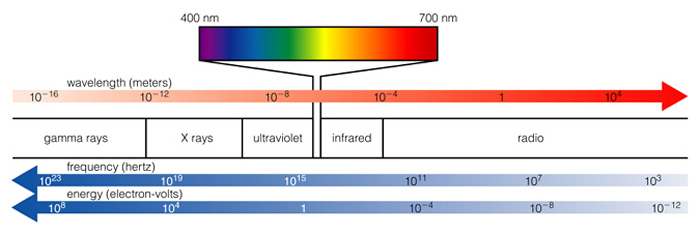 Notice how wavelength increases
in one direction and frequency increases in the other direction, so that
the product of the two is constant, and equals the speed of light.
Notice how wavelength increases
in one direction and frequency increases in the other direction, so that
the product of the two is constant, and equals the speed of light.
We said earlier that less
massive things display wave-like properties, but it is more accurate to
say less energetic things display wave-like properties. As it happens,
photons
with higher frequency also have higher energy. So photons
at the long-wavelength (low energy) end of the spectrum (e.g. radio or
infrared) behave mostly like waves, but at the short-wavelength (high energy)
end of the spectrum (X rays and gamma rays) behave more like particles.
The high energy of X rays explains why they can pass through solid objects
like people, so that you can see your bones and internal organs in an X
ray photograph.
You should memorize the wavelength,
frequency and energy order of these forms of light, e.g.
-
order from long to short wavelengths:
radio, infrared, visible, ultraviolet, X rays, gamma rays
-
order from high to low frequency: gamma rays, X rays, ultraviolet, visible, infrared, radio
- order from high to low energy: gamma rays, X rays, ultraviolet, visible, infrared, radio
Note that the order for energy and frequency is the same.
This is called the electromagnetic
spectrum. A spectrum generally is the splitting of light
into its colors, but our eyes can see only visible light colors (red through
violet). Visible light is only a small part of the entire spectrum. The
other terms, like infrared, or ultraviolet, can also be referred to as colors of light, even though they may be invisible colors (to our eyes). We
have to develop techniques to detect these invisible colors (e.g. radio antennas
can pick up radio waves, IR goggles can detect infrared, photographic film
can detect X-rays, etc.)
Lecture Question
#1
When we raise the temperature
of a body, it first glows only in the infrared, being dark to our eyes. But when hot enough it starts glowing a dull red that we can see, then bright red,
then yellow, then blue.
 |
We
can see this effect in a flame, where the flame is blue at the bottom,
where it is hot, but it cools as it rises and becomes white-yellow,
then red where it is coolest.
|
If we take such a body and spread
out its light into a spectrum, what would we see? We would see a smooth
rainbow of colors, but the brightest part of the rainbow would shift to higher
frequencies (bluer colors) as we raise the temperature. This smoothly
varying spectrum is called a continuum
spectrum because it is made up of all possible frequencies.
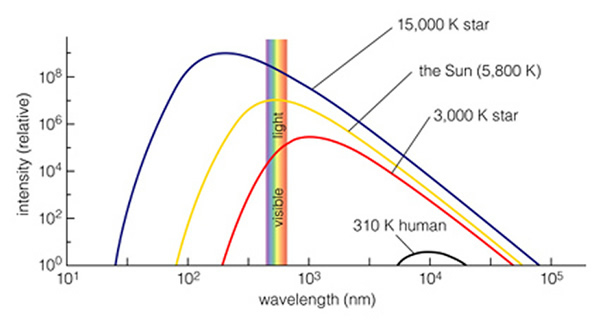
Above is a plot of continuum spectra
for different temperatures. You can see the narrow part of the overall
spectrum that corresponds to what our eyes can detect. The spectrum for a human body is far to the right (longer wavelengths) of what our eyes can see. That is why we
cannot see humans glowing.
 |
Unless,
of course, we have an infrared camera. Notice that these people are
glowing, except the person on the left has cool fingers!
|
A 3,000 K object (like a star),
can be seen to glow with much more red light than blue light, so it looks
red. The Sun, at 6,000 K, peaks exactly in the middle of the visible,
so we see all colors equally (so the sunlight looks white). An even
hotter star, at 15,000 K, will appear blue, but its real peak is in the ultraviolet
where we cannot see. There is a quantitative relationship between wavelength
of light and temperature, called Wein's Law. The relationship is wavelength
proportional to 1 / temperature (lambda ~1/T). So if you double the temperature
of something, you halve the wavelength.
Such continuum spectra are what
we see from objects that are in equilibrium at some temperature. From
the spectrum we can determine the temperature, so we have no problem telling
the temperature of stars. But the light contains MUCH more information
than this. For example, consider a green leaf. The leaf is green
because it absorbs all colors of light except
green. The green light is reflected, and that is why we see the leaf's
color as green. Consider the sky. It appears blue because the
air reflects (or a better term is scatters) the blue light, letting the other
colors, especially red, pass through. We say it is transparent to red,
but only partially transparent to blue. Of course, air is clear, so
it is very transparent to all colors, but if you see the setting Sun through
a lot of air, you can see that the Sun becomes red. That is because
almost all of the blue light doesn't make it to our eyes--it is all scattered
away.
Not just air, but any gas is good
at absorbing (or scattering) some colors
and good at transmitting others. In fact, if you heat a gas it emits
the same colors that it absorbs. If you split the colors of a glowing
gas, such as a candle flame or a neon sign, with a prism or diffraction grating,
you will see that it emits only in certain narrow bands of color. These
bands are called spectral lines.
Here are some characteristics of spectral lines:
- Spectral
lines are seen when light is passed through a slit, then expanded
into a spectrum. A device for doing this is called a spectroscope.
- Absorption
lines are dark lines due to "missing" frequencies
- Emission
lines are bright lines due to extra emission at some frequencies
- Glowing gas shows emission
lines, but if you put this same gas in front of a bright source of light,
the same lines appear in absorption.
- For gas of different composition,
the spectral lines appear in different places, unique for each element.
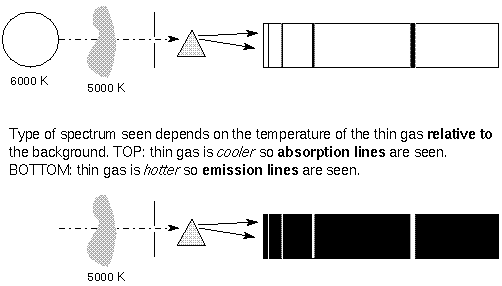
Spectral Lines and the Blackbody
Spectrum
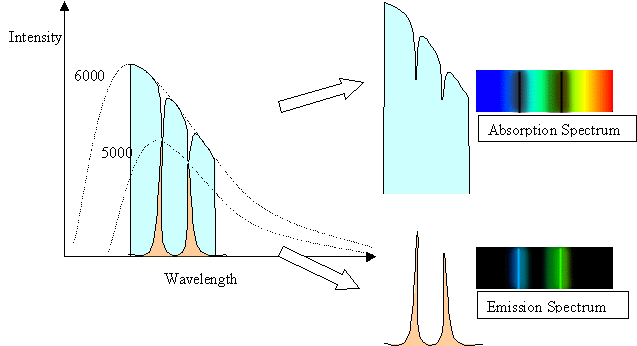
The spectral line intensities are related to the temperature of the body
doing the emitting. In the case of the
5000 K gas in front of the 6000 K background, the background has a normal
Planck Function blackbody
spectrum except where the cooler gas is absorbing it. The
depth of the lines reflect the 5000 K blackbody
spectrum of the gas. In the case of the 5000 K gas with a cool background,
the height or intensity of the
spectral lines reflects the 5000 K blackbody curve of the gas, but only
in the spectal lines. At other wavelengths,
the gas has no emission, and so is dark.
Select the Light
and Spectroscopy tutorial on Astronomy Place web site and go through it.
Lecture
Question #2
Spectral Lines and the Doppler
Shift
When you hear a car or train go
by, you will hear the pitch of the sound go from a high pitch to a low pitch.
That is because the car or train is going a good fraction of the speed of
sound, and the waves "bunch-up" ahead of the vehicle and "spread-out"
behind it. The pitch, or frequency of the waves get higher ahead and lower
behind, and that is what we are hearing. This effect is called the Doppler
effect. By measuring the amount of shift in the pitch, we can measure the
speed of the vehicle.
The same thing happens for light,
but notice that the speed of the object has to be high enough compared
to the speed of light, which means the object has to be moving fast indeed.
In this case, it is not a "pitch" change that we hear, but a color
change that we see.
Fortunately, we can use markers in
the spectrum of light--the spectral lines--to make very accurate measurements
of shifts of spectral lines. From these shifts, we can detect even rather
small velocity changes.
Select the Doppler
Effect tutorial on Astronomy Place web site and go through it.
Lecture Question
#3
When you are finished with these
tutorials, you should know that from starlight we can determine
- the temperature of an object
- the composition of an object
(what it is made of)
- the speed of the object or
parts of the object either toward or away from us
We can also put this information
together to determine other things. If we see star's spectral lines shifting
back and forth we can guess that an object (another star or a planet) is orbiting
it and causing the star to move in a circular path. From the period of
the shifts, we can tell the orbital period of the object. If we know the
mass of the star, we can determine how far the object is from the star.
Or if we know how far the object is away, we can determine the mass of the star.





