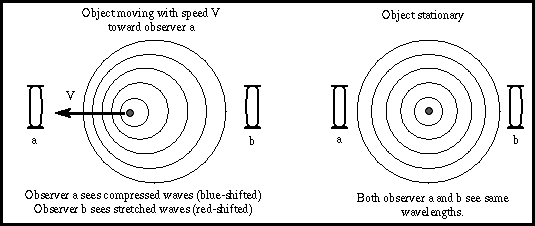
- Spectral lines are seen when light is passed through a slit, then expanded into a spectrum. A device for doing this is called a spectroscope.
- Absorption lines are dark lines due to "missing" frequencies
- Emission lines are bright lines due to extra emission at some frequencies
- Glowing gas shows emission lines, but if you put this same gas in front of a bright source of light, the same lines appear in absorption.
- For gas of different composition, the spectral lines appear in different places, unique for each element.
Spectral Lines and the Blackbody Spectrum
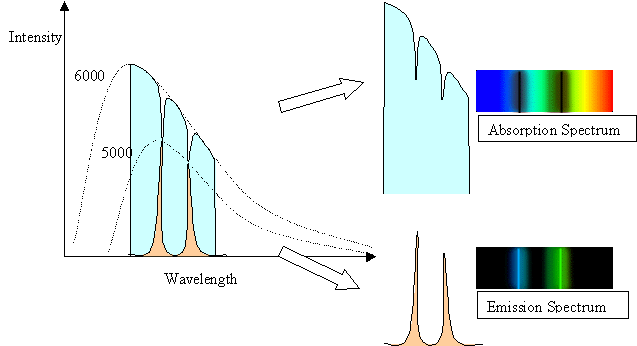
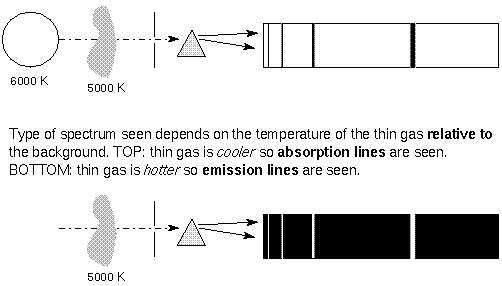
The spectral line intensities are related to the temperature of the
body doing the emitting. In the case of the
5000 K gas in front of the 6000 K background, the background has a
normal Planck Function blackbody
spectrum except where the cooler gas is absorbing it.
The depth of the lines reflect the 5000 K blackbody
spectrum of the gas. In the case of the 5000 K gas with a cool
background, the height or intensity of the
spectral lines reflects the 5000 K blackbody curve of the gas, but
only in the spectal lines. At other wavelengths,
the gas has no emission, and so is dark.
Measuring Spectra
Spectra are measured in wavelength, either in angstrom (1 A = 10-10 m), or nanometer (1 nm = 10-9 m) units. The use of nm is more common in scientific literature, but angstroms are used often in ordinary speech. Visible light ranges from about 4000 A (blue) to 8000 A (red), or 400 nm - 800 nm. We can convert any wavelength to frequency using the fact that the wavelength times the frequency equals the wave speed. For light, that means ln = c. The frequency of 500 nm light isn = c/l = 3 x 108 m/s / 5 x 10-7 m = 6 x 1014 Hz.Converting a wavelength shift to a change in frequency is a little trickier. A wavelength shift of 0.1 nm (1 A) at 500 nm corresponds to a frequency shift ofDn = n - n0 = c/l - c/l0 = c/l02 (l0 - l) = - c/l02 Dl
= - [3 x 108 m/s / (5 x 10-7 m)2] 0.1x10-9 m = -1.2 x 1011 Hz.