| Physics 321 |
Astrophysics II: Lecture #7
|
Prof. Dale E. Gary
NJIT
|
Stellar Interiors -- I
Clusters and Main Sequence
Fitting
We mentioned earlier the
idea of finding distances to stars using spectroscopic parallax.
There is a related method for finding the distance to star clusters, which
is an extremely important thing to know. Why are clusters important?
We can be relatively sure of three things about star clusters, such as
the one below:
-
All of the stars are at nearly
the same distance.
-
All of the stars were born at
nearly the same time.
-
All of the stars have the same
composition,
since they were born from a single cloud (as we will discuss later).

A Globular Cluster, M3
If we look at the H-R diagram
of a star cluster, such as the one below, we see that it is quite different
from the one we made from the nearby stars catalog. This difference
is a very important clue about the way stars evolve.
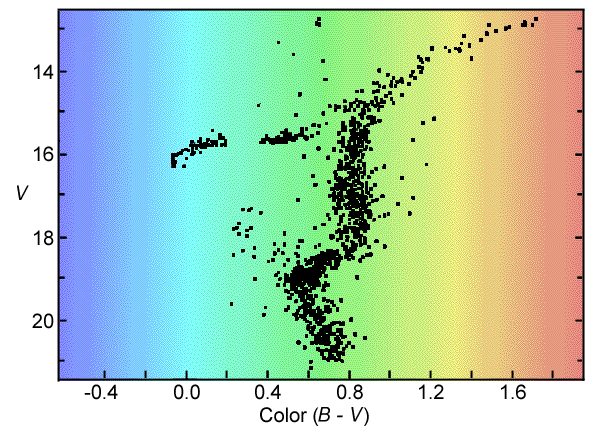
The H-R (color-magnitude) diagram for the globular cluster M3.
We can use the cluster H-R
diagram above to find the distance to the cluster by comparing it with
a calibrated H-R Diagram such as the one we constructed earlier
from the Nearby Stars catalog. Such a comparison uses the technique
of main sequence fitting as illustrated below. Here we have
scaled the cluster diagram (shown in red) to the same scale as the calibrated
H-R diagram, and shifted it keeping the B-V values aligned, until the main
sequences overlap. We can then read the distance modulus directly
off the graph. In this case it is about 15.4, which places the cluster
at about 12 kpc.
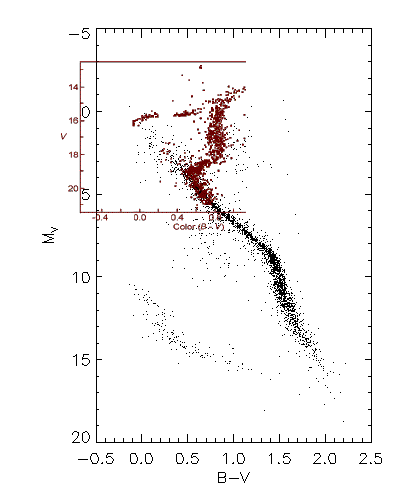
Main Sequence Fitting--comparison of the H-R diagram
of M3 with the calibrated H-R diagram of nearby stars.
We will come back to the
use of cluster H-R diagrams for another purpose, the
determination of the age of the universe! But first we
have to learn something about stellar evolution.
Stellar Interiors
To understand why stars
differ from one another in different parts of the H-R diagram, we have
to look at their basic properties. Every star is a giant sphere
of hot gas (actually a plasma), and certainly those along the main sequence
are pretty much made of the same stuff. The main essential properties
that cause them to differ are pressure
P, temperature T, and composition,
characterized by the mean molecular weight, m.
Let's look at composition, first.
Composition
Stars are made of
relatively simple stuff. By mass, our Sun is 73% hydrogen, 26% helium,
and only 1% of higher Z (atomic number) atoms. We write these quantities
in terms of the mass fraction, as
-
X = mHnH
/
r
= density of hydrogen / total density
-
Y = mHenHe
/
r
= density of helium / total density
-
Z = mZnZ
/
r
= density of everything else / total density
The way that composition
plays a role in stellar structure is mainly through the mean molecular
weight, defined in terms of its inverse as:
where n is the number
density (particles cm-3)
of particles of all types, i.e. n = ne
+ nH + nHe
+ nZ. This is the number density
of electrons plus all of the kinds of atoms. A good approximation
for most star interiors is that the gas is fully ionized. For a fully
ionized gas, how many electrons will there be? Each hydrogen atom
will contribute 1 electron. Each helium atom will contribute 2, and
each higher element will contribute Z (that is, a number equal to the atomic
number of the element). In this case, we have the mean molecular
weight given by:
1/m
= 2 X + 3/4 Y + 1/2 Z
For the Sun, X = 0.73,
Y = 0.26, and Z = 0.01, so 1/m
~ 1.67. The other atoms besides hydrogen and helium are referred
to in astronomy as metals, and the
value of Z is called the metallicity.
Pressure
Pressure is force/unit
area, F / A. The atmospheric pressure at sea level
is about 14 lbs/square-inch, or 10 Pascal, or 1000 millibars (mm of mercury).
Pressure in the interior of a star arises from the weight of the atmosphere
directly above the point--that is, from the force of gravity--balanced
by the outward pressure due to energy release inside the star. Nearly
all stars are static, meaning that they are not expanding or contracting
significantly. Such a state is called hydrostatic
equilibrium, and like all equilibrium situations, it is characterized
by force balance. Consider a column of gas of cross-sectional area
A.
The forces on a section (parcel) of the gas is shown in the figure on the
left:
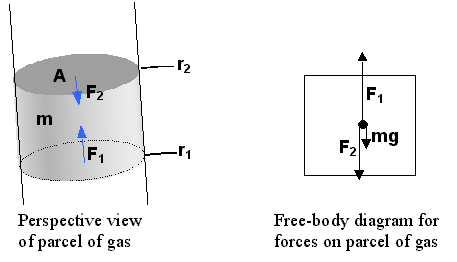
The mass of the parcel is
related to the mass density by r
= m / V = m / A(r2
- r1). From the free-body
diagram it is clear that
F1
= F2 + mg = F2
+ A(r2 - r1)
rg
which can be rearranged to
where DP
is the pressure difference (P1
- P2) on the two ends of the column
section, and Dr
is the radius difference (r1
- r2). Taking the limit as
Dr
approaches zero, we obtain the equation of hydrostatic equilibrium:
There is another relationship
we can obtain between pressure and density (and temperature) called the
equation
of state.
Temperature and the Equation
of State
The temperature,
density, and pressure are not independent, but are joined by the equation
of state, which describes the macroscopic manifestation of particle interactions.
One well-known example of an equation of state is the ideal
gas law:
PV = NkT
where P = pressure, V
= volume, N = number of particles, k = Boltzmann's constant,
and T = temperature. We can write this more simply in terms
of the number density n = N / V as
P = nkT.
Above in equation (1), we saw
that the number density is related to the mass density by
n = r/mmH
so the equation of state becomes
P = rkT
/
mmH
.
(3)
Inserting (3) into (2), and
assuming for the moment that temperature T does not change with
height (an isothermal atmosphere), we obtain an expression for the density:
dr/dr
= - (mmHg
/ kT) r
(4a)
which is a first-order
differential equation with the solution:
r
= ro
exp(-r
/
H)
where H = kT /
mmHg
is called the scale height. and ro
is called the base density since it
is the value of the density at r = 0. Note that if we had
solved for r
in equation (3) and substituted it for r
on the right-hand side of equation (2), we would obtain a similar differential
equation for the pressure:
dP/dr
= - (mmHg
/ kT) P
(4b)
with the solution:
P = Po
exp(-r
/
H).
Therefore, the pressure and
density vary with the same scale height. What is the physical interpretation
of the scale height? It means that the pressure and density drops
with height by the same factor of 1/e = 0.368 for each increase
in height of a distance H. Note that a hot atmosphere (large
T)
has a larger scale height than a cool atmosphere. Also, increasing
gravity lowers the scale height. It is of interest to calculate the
scale height for some atmospheres.
Example
1: Scale height at Earth's surface. H = kT /
mmHg,
where T ~ 300 K, g = 9.8 m/s2,
but what is m?
The atmosphere is mostly (4/5) molecular nitrogen, N2,
which has a mean molecular weight of 28. Using these values, we obtain:
H = kT
/
mmHg
= (1.38x10-23 J/K)(300
K) / [28(1.67x10-27 kg)(9.8 m/s2)]
= 9.0
km
In fact, the Earth's atmosphere
rapidly grows cooler with height, so the scale height is somewhat smaller.
Example
2: Scale height in the photosphere of the Sun. Here, T
= 5770 K, m
~ 0.6, but what is g? Remember that mg is the force
of gravity, which is also
F =
mg
= GMm / R2
so g = GM / R2.
At the surface of the Sun, we would use M = 1.989x1030
kg,
R = 6.96x108
m (from Appendix 7 in the text), so
g = (6.67x10-11
N m2 / kg2)(1.989x1030
kg)/(6.96x108
m)2
= 296 m/s2.
~
30 g
Then,
H = kT
/
mmHg
= (1.38x10-23
J/K)(5770 K)
/ [0.6(1.67x10-27
kg)(296 m/s2)]
= 270
km
Other Equations of State
In the atmospheres
of stars and planets, the ideal gas law is a pretty good approximation.
However, in the interiors of stars some new effects can come into play.
In any star, near the core, especially, the light itself can exert significant
pressure. A photon has momentum p = hn/
c,
and when it is absorbed or reflected it exerts a force
F = dp/dt.
Consequently, electromagnetic radiation has an associated pressure.
It may come as no surprise that the pressure is related to the total radiant
energy flux, which we saw from the Stefan-Boltzmann law was F =
sT4.
One can show that
Prad =
1/3 aT 4
where a = 4s/c
= 7.56591 x 10-16
J m-3 K-4
is the radiation constant. In
this case, the total pressure, equation (3), is replaced by
P = rkT/
mmH
+
1/3 aT 4
(3')
Finally, when relativistic and
quantum effects are included to derive the equation of state, a very different
pressure equation is found that applies to extremely dense states of matter
such as found in white dwarfs and neutron stars. We will look into
this later in the course.


