Gennady Gor: Research
The main focus of the Laboratory for Materials Interfaces is nanoporous materials, solids with pores of 100 nanometers and below. Such materials play a significant role in both nature and technology. Synthetic nanoporous materials are employed in the chemical industry as adsorbents, catalysts and separation membranes, among other uses. Naturally occurring nanoporous materials include coal and shale, key fuels in the production of energy. Another research focus is soot agglomerates, which are not porous, but rather nanostructured materials with features on the same scale as nanoporous solids. We work on the wide spectrum of phenomena related to the interfaces between these nanoporous or nanostructured solids and fluids: fluids adsorption, fluids transport and the propagation of ultrasound in fluid-saturated porous media, to name a few. Our approaches are mainly theoretical; we use various modeling techniques to represent phenomena at the nanoscale: Monte Carlo simulations, molecular dynamics, density functional theory and finite element analysis. However, in 2022 we added experimental research to our portfolio.
The current projects are:
- Elastic Properties of Confined Fluids
- Electrolytes in Nanopores and Electrochemical Actuation
- Morphological Changes of Atmospheric Black Carbon
- Adsorption-Induced Deformation of Nanoporous Materials
- Molecular Modeling of Toxic Compounds
- Modeling of Amino Acids at Mineral Interfaces
- Ultrasound Propagation in Electrochemical Devices
- Materials Properties of Separators in Li-Ion Batteries
- New Methods for Characterization of Porous Materials
The group, led by Ella, recorded a nice overview of the group research in spring 2024.
This recording of a presentation made for undergraduate students in 2020 also gives a short overview of some of the research projects. Another video highlights Jason Ogbebor's undergraduate research experience. (After graduation from NJIT in 2023 Jason continued his research as a Ph.D. student at MIT).
Note for potential graduate and undergraduate researchers: we are always looking for strong candidates to join the group. The list of the possible projects is not limited to the ones above. Interested candidates should email to Dr. Gennady Gor gor@njit.edu with a short cover letter and a CV. Although most of the projects involve collaborations with experimental groups at NJIT and beyond, our research is mostly theoretical and computational. Thus strong math skills is a necessary condition to join the group, programming experience is a plus.
Elastic Properties of Confined Fluids
Compressibility of a fluid in a porous medium determines the response of the medium to mechanical loads, and elastic waves propagation in particular. If the pores of the medium are in the nanometer range, many thermodynamic properties of the fluid confined in such pores are altered, and the fluid compressibility is not an exception. We study the compressibility of simple and complex fluids in confinement using both molecular simulations and experiments relate in order to understand the properties of fluid-saturated nanoporous media (such as hydrocarbons bearing shales).
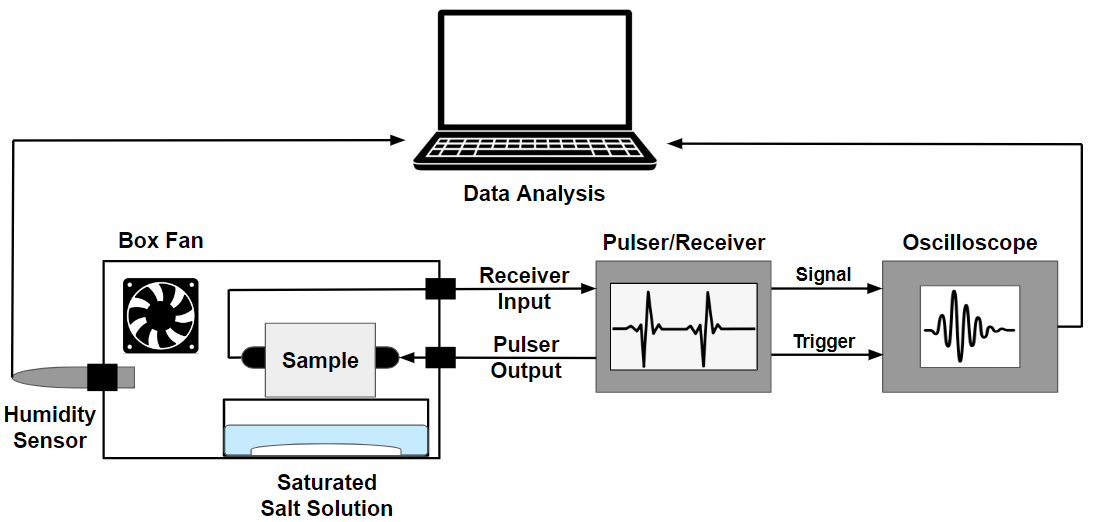
Funding: National Science Foundation (CBET-2128679 and CBET-2344923), New Jersey Institute of Technology (Seed Grant)
Publications: Ogbebor, J.; Valenza, J. J.; Ravikovitch, P. I.; Karunarathne, A.; Muraro, G.; Lebedev, M.; Gurevich, B. Khalizov, A. F.; Gor, G. Y."Ultrasonic Study of Water Adsorbed in Nanoporous Glasses" Phys. Rev. E 2023, 108, 024802. DOI: 10.1103/PhysRevE.108.024802
Flores Roman, S. A.; Barbosa, G.; Gor, G. Y.
"Elasticity of Confined Simple Fluids from an Extended Peng-Robinson Equation of State"
Ind. Eng. Chem. Res. 2023, 62, 8972-8980, DOI: 10.1021/acs.iecr.3c00549
Dobrzanski, C. D.; Gurevich, B.; Gor, G. Y.
"Elastic Properties of Confined Fluids from Molecular Modeling to Ultrasonic Experiments on Porous Solids" Appl. Phys. Rev. 2021, 8, 021317, DOI: 10.1063/5.0024114
Maximov, M. A.; Gor, G. Y. "Molecular Simulations Shed Light on Potential Uses of Ultrasound in Nitrogen Adsorption Experiments", Langmuir
2018, 34(51), 15650-15657, DOI: 10.1021/acs.langmuir.8b02909.
Gor, G. Y.; Gurevich, B. "Gassmann Theory Applies to Nanoporous Media", Geophys. Res. Lett., 2018, 45(1), 146-155, DOI: 10.1002/2017GL075321, preprint is available at arxiv.org arXiv:1710.05216 [physics.geo-ph]
Researchers: Jason Ogbebor, Ashoka Karunarathne, Santiago Flores Roman Collaborators: Alexei Khalizov (NJIT), Boris Gurevich (Curtin University, Australia), Maxim Lebedev (Curtin University, Australia), John Valenza (Exxonmobil), Peter Ravikovitch (Exxonmobil)
On the news: NJIT Research Team Discovering How Fluids Behave in Nanopores with NSF Grant.
Electrolytes in Nanopores and Electrochemical Actuation
When salts dissolve in water, they form electrolytes. Near a charged solid surface ions in an electrolyte redistribute, forming an electric double layer (EDL). EDL plays an important role in geochemical and biological processes and is utilized in technologies for electrochemical energy storage. While the structure and properties of EDL are well understood for an EDL near a planar surface, understanding of EDL in nanoporous materials is poor. These nanoporous materials are of interest for electrochemical energy storage applications, which motivates this project. In particular EDL formation in nanoporous materials causes mechanical stresses and lead to materials deformation. This research project aims to quantify the formation of EDL in aqueous solutions of electrolytes in nanoporous materials and relate it to stresses and strains in these materials.
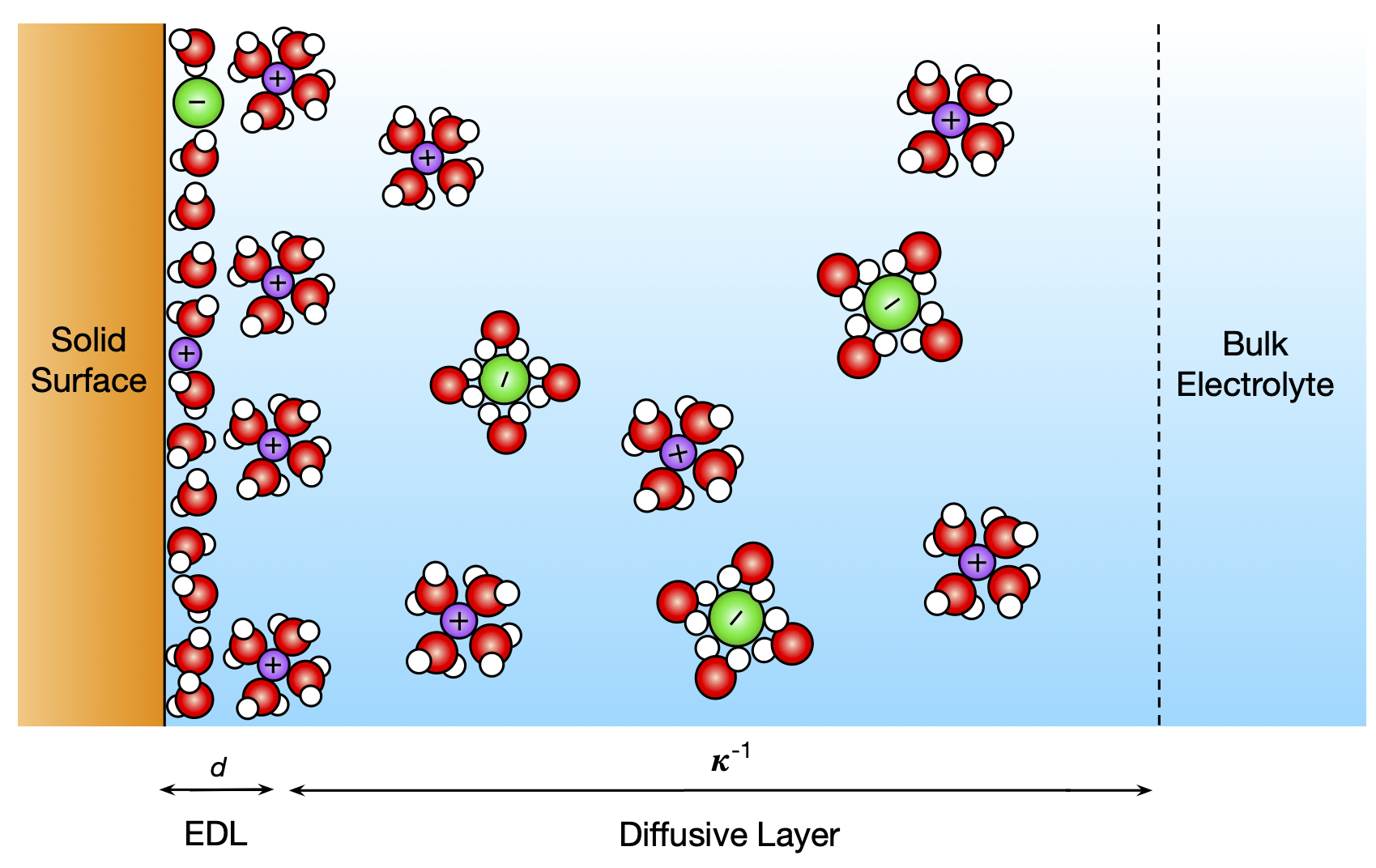
Funding: National Science Foundation (CBET-2234028)
Researchers: Andrei Kolesnikov Collaborators: Patrick Huber (TUHH, Germany), Prof. Michael Froba (University of Hamburg, Germany)
On the news: NJIT Research on Nanoporous Materials Would Make Electronics Stronger, Safer.
Morphological Changes of Atmospheric Black Carbon
Soot is a major environmental pollutant with impacts ranging from air quality and human health to climate. The extent of these impacts depends on the microstructure of soot nanoparticles and their surface properties. The soot microstructure is complex, with nanoparticles being fractal aggregates of graphitic spherules mixed with organic and inorganic combustion products or other atmospheric chemicals. On top of it, soot nanoparticles often change structure when interacting with chemicals adsorbed on their surface. The main goal of this project is to develop a molecular-based model for soot nanoparticles restructuring (Gor's group) and verify it against experimental measurements (Khalizov's group).
Funding: National Science Foundation (AGS-2222104), New Jersey Institute of Technology (Seed Grant)
Publications: Ivanova, E. V.; Emelianova, A.; Khalizov, A. F.; Gor, G. Y. "Molecular Simulation of Benzene Adsorption in Graphitic and Amorphous Carbon Slit Pores"
J. Chem. Eng. Data 2022, 67, 7, 1765-1778, DOI: 10.1021/acs.jced.2c00063
Ivanova, E. V.; Khalizov, A. F.; Gor, G. Y. "Kinetic Model for Competitive Condensation of Vapor between Concave and Convex Surfaces in a Soot Aggregate"
Aerosol Sci. Technol. 2021, 55(3), 302-315, DOI: 10.1080/02786826.2020.1846677
Chen, C.; Enekwizu, O. Y.; Fan, X.; Dobrzanski, C. D.; Ivanova, E. V.; Ma, Y.; Gor, G. Y.; Khalizov, A. F. "Single parameter for predicting the morphology of atmospheric black carbon", Environ. Sci. Technol., 2018, 52(24), 14169-14179, DOI: 10.1021/acs.est.8b04201
Researchers: Ella Ivanova, Egor Demidov Collaborators: Alexei Khalizov (NJIT), Nicole Riemer (UIUC)
Adsorption-Induced Deformation of Nanoporous Materials
When a solid surface accommodates guest molecules, they induce noticeable stresses to the surface and cause its strain. Nanoporous materials have high surface area and, therefore, are very sensitive to this effect called adsorption-induced deformation. In recent years, there has been significant progress in both experimental and theoretical studies of this phenomenon, driven by the development of new materials as well as advanced experimental and modeling techniques. Also, adsorption-induced deformation has been found to manifest in numerous natural and engineering processes, e.g., drying of concrete, water-actuated movement of non-living plant tissues, change of permeation of zeolite membranes, swelling of coal and shale, etc. Our goal is to develop a quantitative molecular-based model for this phenomenon.

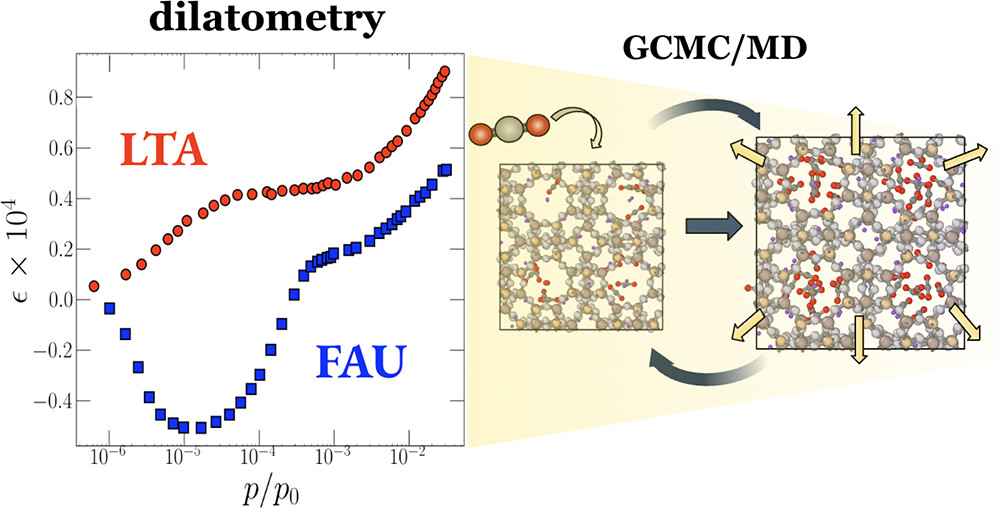
Funding: National Science Foundation (CBET-1944495)
Publications: Gor, G. Y.; Scherer, G. W.; Stone, H. A. "Bacterial Spores Respond to Humidity Similarly to Hydrogels" PNAS
2024, 121 (10) e2320763121, DOI: 10.1073/pnas.2320763121
Emelianova, A.; Balzer, C.; Reichenauer, G.; Gor, G. Y. "Adsorption-Induced Deformation of Zeolites 4A and 13X: Experimental and Molecular Simulation Study" Langmuir 2023, 39, 32, 11388-11397 DOI: 10.1021/acs.langmuir.3c01248, [ACS Editor's Choice]
Yurikov, A.; Lebedev, M.; Gor, G. Y.; Gurevich, B. "Sorption-Induced Deformation and Elastic Weakening of Bentheim Sandstone", J. Geophys. Res. Solid Earth, 2018, 123(10), 8589-8601 DOI: 10.1029/2018JB016003
Gor, G. Y.; Huber, P.; Bernstein, N. "Adsorption-Induced Deformation of Nanoporous Materials - a Review", Appl. Phys. Rev., 2017, 4, 011303, DOI: 10.1063/1.4975001
Researchers: Alina Emelianova, Andrei Kolesnikov Collaborators: Oskar Paris (Montanuniversity Leoben, Austria), Gudrun Reichenauer (ZAE Bayern, Germany), Patrick Huber (TUHH, Germany)
Molecular Modeling of Toxic Compounds
In some cases molecular simulations can be used as an alternative to experiments. This is the case in particular when the chemicals of interest are extremely toxic, and experimental measurements are dangerous. Examples include studies of chemical warfare agents, as well as some of the industrial compounds (e.g. isocyanates).
Several organophosphorus compounds, such as G-agents sarin and soman have been synthesized during the World War II to be used as chemical warfare agents (CWA). Despite the 1993 Chemical Weapons Convention, that outlaws the production and use of chemical weapons and their precursors, the use of CWA by terrorists still remain a threat. Thus there is a need to develop processes for chemical protection, capture and decontamination of CWAs. These studies require knowledge of thermodynamic properties of CWAs, in particular quantitative predictions of phase equilibrium of CWA in the presence of adsorbents. Due to the extreme toxicity of CWAs, experiments with them are very limited, and many of the experiments are made with their less toxic simulants. We employ molecular simulations for both CWAs and simulants. Modeling data for simulants can be readily verified experimentally, and justify the use of modeling data for CWAs for predictions as an alternative to experiments.
Read more about Dr. Gor participation in MSEE here and about the grant supporting this research here
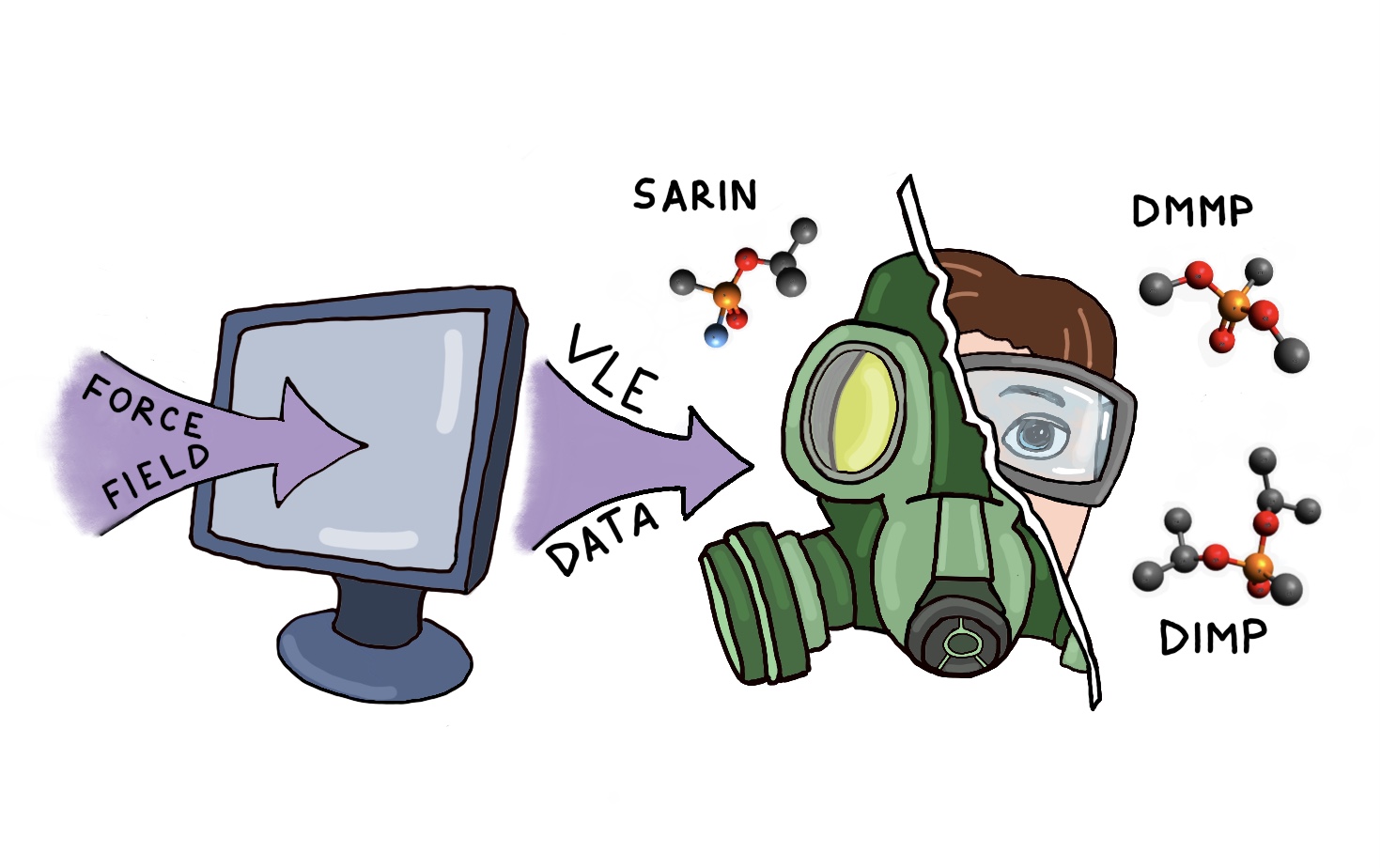
Funding: MSEE URA Grants (DTRA).
Publications: Ivanova, E. V.; Gor, G. Y. "Molecular Dynamics Predictions of Viscosity for Organophosphorus Liquids" AIChE J. 2023, e18235 DOI: 10.1002/aic.18235, [Invited Paper for the 2023 Futures Issue]
Ivanova, E. V.; Vasudevan, A.; Senyurt, E. I.; Schoenitz, M; Khalizov, A. F.; Dreizin, E. L.; Gor, G. Y. "Surface Tension of Organophosphorus Compounds: Sarin and its Surrogates"
Langmuir 2023, 39, 15, 5569-5578, DOI: 10.1021/acs.langmuir.3c00460
Emelianova, A.; Reed, A.; Basharova, E. A.; Kolesnikov, A. L.; Gor, G. Y. "Closer Look at Adsorption of Sarin and Simulants on Metal-Organic Frameworks"
ACS Appl. Mater. Interfaces 2023, 15, 14, 18559-18567 DOI: 10.1021/acsami.3c02713
Emelianova, A.; Gor, G. Y. "Molecular Simulations of Vapor-Liquid Equilibrium of Isocyanates" J. Phys. Chem. B 2021, 125, 45, 12528-12538, DOI: 10.1021/acs.jpcb.1c07132;
Emelianova, A.; Basharova, E.; Kolesnikov, A; Villaseco Arribas, E.; Ivanova, E. V.; Gor, G. Y. "Force Fields for Molecular Modeling of Sarin and its Simulants: DMMP and DIMP" J. Phys. Chem. B 2021, 125, 16, 4086-4098, DOI: 10.1021/acs.jpcb.0c10505
Researchers: Liza Basharova, Ella Ivanova, Allen Reed, Matthew Stickles Collaborators: Edward Dreizin (NJIT), Mirko Schoenitz (NJIT)
Modeling of Amino Acids at Mineral Interfaces
We are studying behaviour of various amino acids to better undestand and fine-tune additives to toothpaste. NJIT highlighted this research in a news item, please read "Chemical Engineering Solves Problems with Computer Simulation". A video below also provides an idea about this project.
Funding: Colgate-Palmolive
Researchers: Alina Emelianova Collaborators: Andrei Potanin (Colgate-Palmolive), Tatiana Brinzari (Colgate-Palmolive)
Ultrasound Propagation in Electrochemical Devices
Modern electrochemical energy storage systems, such as lithium-ion batteries are significantly more complex than the old ones, like alkaline or lead-acid batteries. To maintain proper operation of these systems, it is necessary to monitor a number of parameters representing their "health". Measuring electrical characteristics, such as voltage and current, appears insufficient for such monitoring. Recent experiments showed a correlation between the batteries' health and the speed of ultrasonic waves propagation through them, but, most of research in this area is rather qualitative. We perform a series of ultrasonic studies of electrochemical devices and utilize these data to develop a rigorous physics-based theory of ultrasonic wave propagation in electrochemical energy storage systems.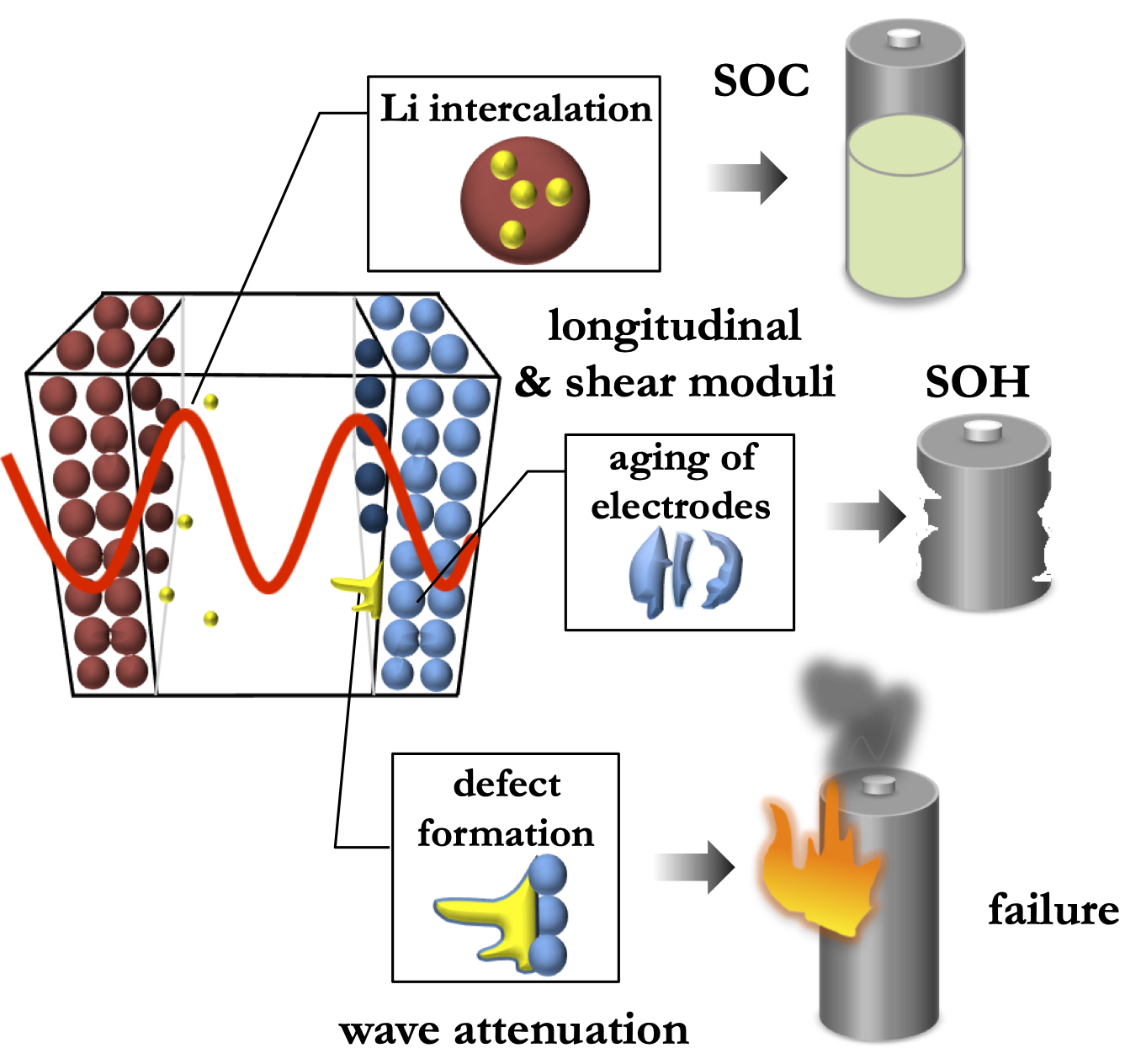
Funding: NJIT Seed Grant
Researchers: Gunel Nabiyeva Collaborators: Alexei Khalizov (NJIT), Craig Arnold (Princeton University)
Materials Properties of Separators in Li-Ion Batteries
Lithium-ion batteries (LIBs) is the leading solution for electrical energy storage, which provide the highest energy and power per unit mass. Although it is already a well-developed technology, it still has one weak point - safety. A lithium-ion battery failure may cause thermal runaway; and the battery can catch on fire. While the performance characteristics of the batteries (e.g. specific power or specific energy) are determined by the electrode materials, the battery safety relies on the separator. LIB separators are typically made of porous semicrystalline polypropylene. Recent experiments showed that, when polypropylene separators are immersed in carbonate solvents used in LIBs, the separators mechanical properties are significantly reduced. The extent of the observed reduction is unexpected, and the physical mechanism is unclear. We aim to elucidate this mechanism on the molecular level to provide a path towards the development of porous polymeric membranes with improved mechanical properties.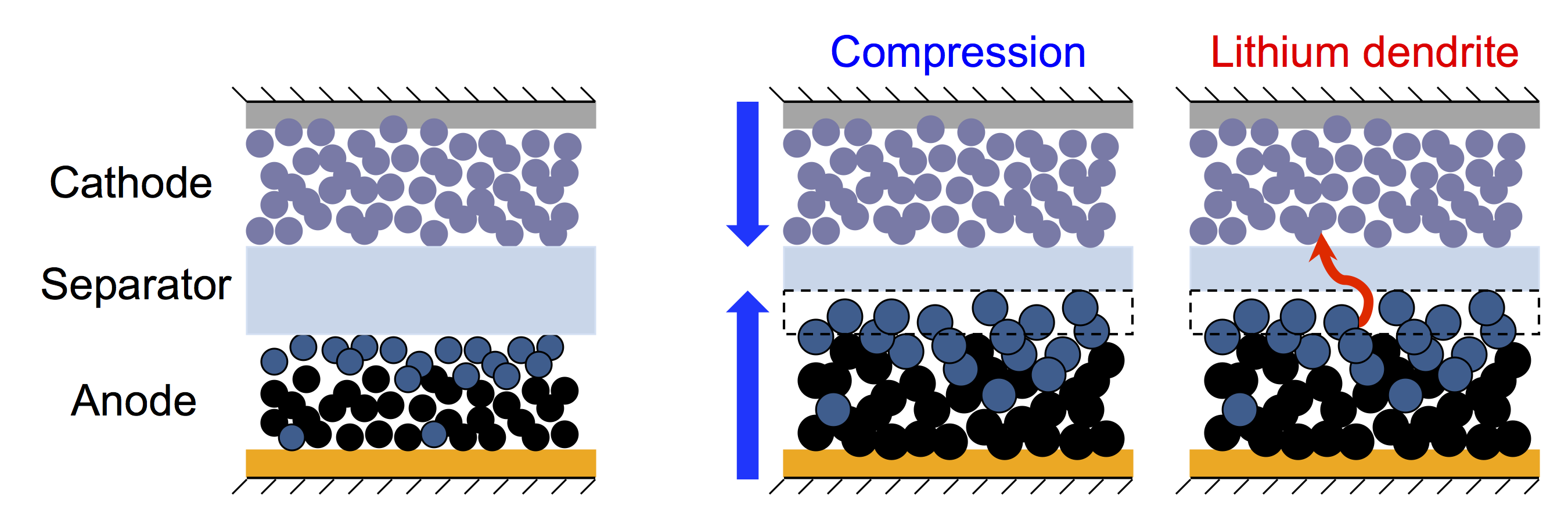
Funding: NJIT Seed Grant
Publications: Maksimov, A. V.; Molina, M.; Maksimova, O. G.; Gor, G. Y. "Prediction of Swelling of Polypropylene Separators and its Effect on the Lithium-Ion Battery Performance" ACS Appl. Polym. Mater. 2023, 5, 3, 2026-2031, DOI: 10.1021/acsapm.2c02074
Researchers: Marcos Molina Collaborators: Craig Arnold (Princeton University), Andrew Maximov (Cherepovets State University, Russia)
New Methods for Characterization of Porous Materials
Porous materials have myriad of applications. Nowadays, materials are carefully tailored for each application. Materials scientists and chemist need precise tools for characterization of the synthesized samples, to determine the surface area, pore morphology, pore size distribution, etc. Gas adsorption has been utilized for this purpose. We develop theoretical models of gas adsorption in porous materials, as well as employ new phenomena for characterization purposes (such as ultrasound or adsorption-induced deformation).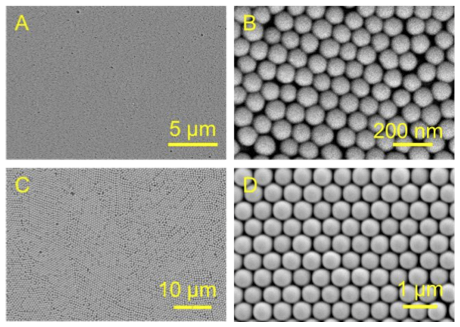
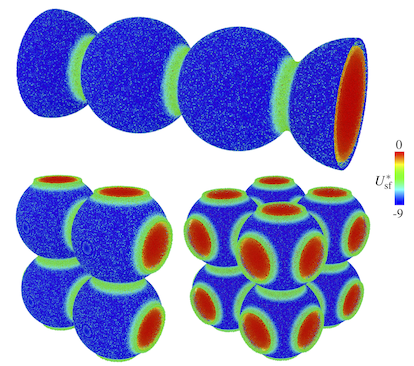
Publications: Maximov, M. A.; Molina, M.; Gor, G. Y. "The Effect of Interconnections on Gas Adsorption in Materials with Spherical Mesopores: a Monte Carlo Simulation Study" J. Chem. Phys. 2021, 154, 114706 DOI: 10.1063/5.0040763;
Maximov, M. A.; Galukhin, A. V.; Gor, G. Y. "Pore Size Distribution of Silica Colloidal Crystals from Nitrogen Adsorption Isotherms" Langmuir 2019, 35(47), 14975-14982, DOI: 10.1021/acs.langmuir.9b02252;
Galukhin, A. V.; Bolmatenkov, D.; Emelianova, A.; Zharov, I.; Gor, G. Y. "Porous Structure of Silica Colloidal Crystals" Langmuir 2019, 35(6), 2230-2235, DOI: 10.1021/acs.langmuir.8b03476
Researchers: Marcos Molina, Alina Emelianova Collaborators: Andrey Galukhin (Kazan University, Russia), Ilya Zharov (University of Utah)