Surface Charges in soils
Permanent Charge
In clays and other minerals different charged ions in crystal structure are substituted during the formation of the mineral.
(Al +3 for Si +4 ; Mg +2 for Al +3, Fe +2 for Al +3)
pH Dependent Charge
At low pH, soil charge becomes more positive.
At high pH, negative charge increases.
Overall charge is usually negative.
Soil Organic Matter usually has a negative charge due to presence of carboxyl, phenolic groups:
Charge on soil particles affects:
¨ mobility of inorganics in soil
¨ mobility of organics in soil
¨ soil buffering of pH changes
Range of composition of humic material:
45-55% Carbon
30-45% Oxygen
3-6% Hydrogen
1-5% Nitrogen
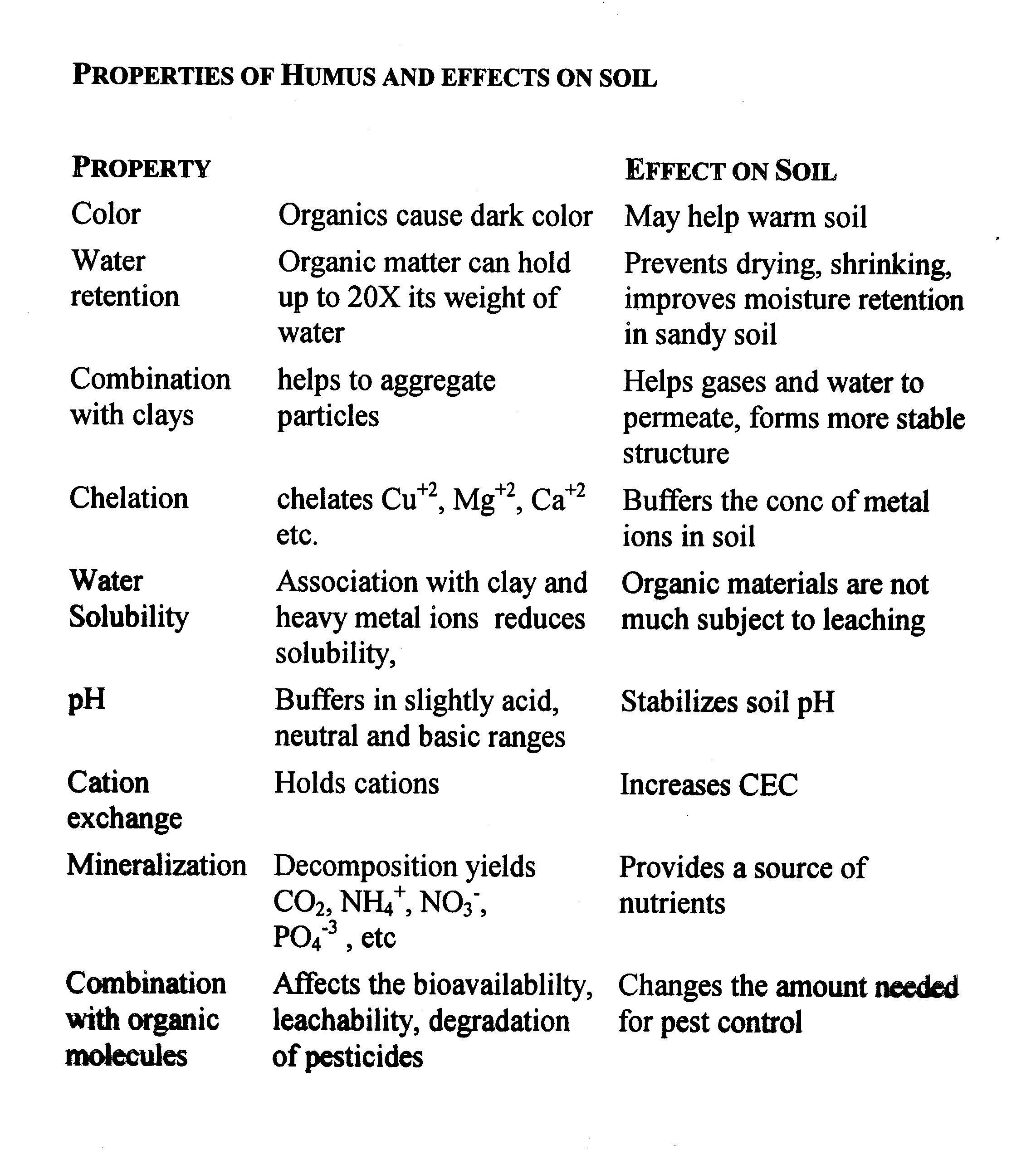
Significant amount of humus exists as colloidal particles
Generally carries a negative charge and has very high surface area.
Sorption Properties of Soil Organic Material
Heavy metals
An element in the soil may exist in three forms :
Fixed, adsorbed, and dissolved.
Cation Sorption:
Like
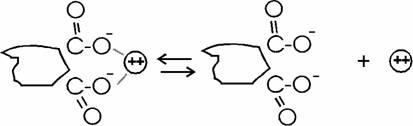
layer silicates, humic substances also have a surface
negative charge, because of dissocation of
![]() and
OH groups
and
OH groups
Sorbed ions can be exchanged, depending on relative concentrations
Total amount of sorbed cations is defined as the cation exchange capacity CEC -- CEC is mostly defined by the clay and SOM content of soil.
The amount exchanged depends on the distribution coefficient:
Kd = Cs / Cw (Conc in soil /Conc in water)
These Kd vary with ion and with different types of soils:
The most strongly sorbed
is the ion with the smallest hydrated radius and the highest charge: For example:
Ba+2 < Ca+2 < Mg+2 and Cs+1 <
K+1 <Na+1 < Li+2
Complex formation in aqueous phase : Formation of a complex will tend to make the cation more leachable.
Anion
Sorption
Most soil particles are negatively charged, but some anions are also bound:
Metal Oxides--
M-O-H in equilibrium with soil water:
At higher pH values MOH + OH- Û MO- + H2O
At
lower pH MOH + H+
Û MOH2+
The edges of Aluminosilicate mineral grains have some atoms which are not fully coordinated, so they can also behave in the same manner as MOH.
Soil Organic
Matter (SOM) has some functional groups which form positive sites: e.g.
R-N-H3+
Anion exchange capacity of soil materials is much lower than the CEC--on order of 20 x less.
Ion exchange is stiochiometric and reversible.
Extent of fixation, sorption of positive and negative ions, complex formation and the kinetics of the sorption reactions all affect the the rate of movement of an element through soil.
¨ Higher surface area decreases the movement of elements
¨ Higher pH will decrease cation mobility
¨ Anion mobility is decreased in high clay soil, and in high metal oxide content soils.
¨ Higher pH will increase anion mobility
Acid base reactions in soil
Sources of soil acids:
¨ Organic acids from microbial oxidation of plant material
¨ Oxidation of H2S from anerobic decomposition of biomass
¨ Microbial oxidation of NH4+ from fertilizer
¨ Phosphate fertilizers
¨
Acid cat clay from weathering of
¨ Acid rain
¨
Dry acid deposition
Soil pH--Soil is a buffer
Materials responsible for soil buffering
¨ SOM
¨ aluminosilicate minerals
¨ oxides and hydroxide minerals
¨ carbonates.
soil
pH Ranges
| Soil
Type | pH | Soil
Type | pH |
| Very strongly acidic | 4.5 - 5 |
Neutral | 6.5-7.4 |
| Strongly Acidic | 5 - 5.5 |
Mildly Alkaline | 7.4 - 7.9 |
| Moderately Acidic | 5.5 - 6 |
Moderately Alkaline | 7.9 - 8.4 |
| Mildly Acidic | 6 - 6.5 | Strongly Alkaline | 8.4 - 9 |
Salt
affected soils
Sorption
of Organic Compounds in Soil
q Molecular Size
q Hydrophobicity
q Molecular Charge
q Hydrogen Bonding
q Coordination
Soil Solution
In capillaries and in films--not easy to sample
Dissolved ions:
Agriculture
Importance of soil to agriculture
Pesticides: Federal review policies for agricultural pesticides
Delaney
Clause as related to pesticides:
Plant
nutrients
Calcium
Magnesium
Sulfur
Nitrogen
Phosphorous
Potassium
Micronutrients
Fertilizers
Wastes
and pollutants
Soil
Erosion
Biochemistry--Genetic
engineering and the green revolution
Contamination
of Food by Agricultural Practice