Order is opposite for reverse phase.
14. In a normal phase LC separation, nitrobenzene had a retention time of 28.0 min, while an unretained compound eluted in 0.9 min. The mobile phase was a 50:50 mixture of chloroform and hexane. What mixture of chloroform and hexane will reduce the k’ to 9.0.
Initial k'1 = (28.0-0.9)/0.9 = 30
P Hexane = 0.1, P toluene = 2.4, from table 4.5
P of initial eluent = 2.4 x 0.5 + 0.1 x 0.5 = 1.25 = PI
Calculate P2 (Eq 4.21)
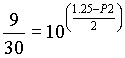 ,
and P2 = 2.29
,
and P2 = 2.29
Let x = fraction of Toluene
2.29 = 2.4(x) + 0.1(1-x)
x = 0.95, so mixture should be 95% toluene, 5% hexane.
15. The following data were obtained for a separation using a 0.5 mm id, 10 meter open tubular column:
| Retention time (min) | Peak base width (min) | |
| Methane | 2.0 | |
| Cyclohexane | 8.0 | 0.65 |
| Methylcyclohexane | 8.17 | 0.72 |
| Toluene | 10.2 | 0.95 |
a) Draw the chromatogram and label the peaks
b) Calculate the number of peaks for each compound
c) Calculate the capacity factor for toluene
b)
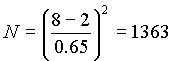 for cyclohexane, 1175 for methylcyclohexane
and 1192 for toluene
for cyclohexane, 1175 for methylcyclohexane
and 1192 for toluene
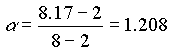 and k' =3
and k' =3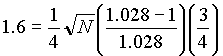
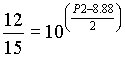 and P2 = 8.69
and P2 = 8.69