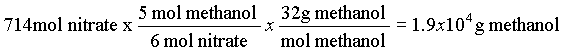
1. from Ksp find conc of Ca which will cause a precipitate.
Ksp = 4.47 x 10-9 = [Ca2+][CO32-]
4.47 x 10-9 = [Ca2+][1.00 x 10-4]
[Ca2+] = 4.47 x 10-5
40 mg Ca+2 /L is 1 x 10-3 M which is pretty much all tied up as CaT-.
Ca+2 + HT2- ® CaT- +H+
for this reaction K = [CaT-][ H+] / [Ca+2][ HT2-]
Where K = 7.7 x 10-3
[CaT-] = 1 x 10-3
[ H+] = 1 x 10-9
[Ca+2] = 4.47 x 10-5
Solving gives [ HT2-] = 2.89 x 10-6
This very low value means that an exceedingly low excess of NTA is required to chelate all the Ca present. So 1 x 10-3 mol /l of NTA is needed, or 1 mol in 1000 l.
3. Conversion to biomass, which settles, and conversion to CO2.
5. Water molecules are selectively sorbed in the membrane and are selectively passed through the membrane. Ions are excluded.
6. 2000 l/day x 50 mg/l of N is 1.00 x 104 g N which is 714 mol N (and 714 mol NO3-). from the equation (8.9.6) 5 mol methanol reacts with 6 mol nitrate.
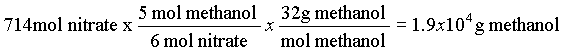
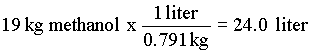
12. Boiler water is most affected by hardness, because hard water will cause scaling and clog boiler tubes.
14. NTA removes Cu by keeping it in solution as a complex, not by a precipitation.
18. Sequential use means that the water is used several times for purposes which need successively lower quality. It must be kept in mind, so nothing is added in one stage which will hamper the next use.