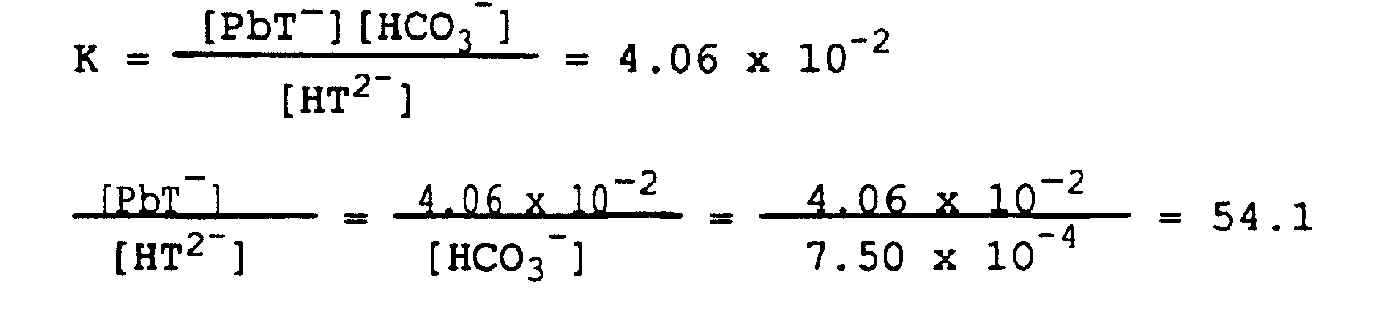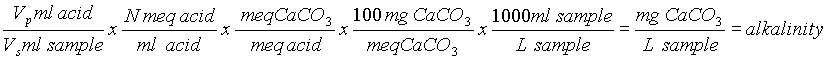
Chapter 3
1. Normality is expressed in equivalents/liter, similar to Molarity, but taking into account the fact that some acids can neutralize more than one mole of OH- per mole of acid. (Think of 1 M HCl and 1 M H2SO4, for example. 1 M H2SO4 will neutralize twice as much OH- and is therefore, 2 N. 1 M HCl is 1 N.) Now, the phenolphthalein end point is at pH 8, and therefore any CO3= will be converted to HCO3-. One mole of H+is consumed by each mole of CO3=. So CO3= also has one equivalent per mole in this reaction.
simplifies to (Vp x Nx 105)/Vs
2. In 100 lb of C12H22O11 there are 4.54x107 mg or 1.33x105 mmol of C12H22O11. Water saturated with oxygen at 25 C contains 8.3 mgO2/liter. The oxidation equation is C12H22O11 + 12 O2--> 11H2O + 12 CO2
12 x 1.33x105 mmol of oxygen is needed.
Volume of water containing enough oxygen to degrade all the sugar is 6.2 x 106 liters. (not all the steps are shown here--be sure to fill them in!)
5. Calcium carbonate dissolves when HCl is injected according to the reaction:
CaCO3 + HCl ® Ca2+ + Cl- + HCO3-
So the hardness = [Ca2+] = 1x 10-3 M. and the alkalinity = [HCO3-] = 1x 10-3 eq/liter.
When HF is injected the result is more complicated. The CaCO3 will dissolve by a similar reaction until the solubility of CaF2 is exceeded.
CaCO3 + HF ® Ca2+ + F- + HCO3-
Then the excess CaF2 will precipitate. To make formulating the equation simpler, let's take this in two steps--let all the HF dissolve the CaCO3, yielding a solution which is 1 x 10-3M in both ions. Then let the excess precipitate. (These happen simultaneously in reality, but the end result is the same.)
Let x be amount of Ca2+ which has precipitated at equilibrium. The amount of F- which precipitates is twice that of Ca2+ (because we are forming CaF2) so it is 2x. Plugging into the Ksp equation
Ksp = [Ca2+] [F-]2 = (1x 10-3 - x)(1x 10-3 - 2x)2 = 3.90 x 10-11
This is a cubic equation and not easy to solve. You can use a computer program like Mathcad to do it, or make an estimate and do some successive approximations.
The answer is x = 3.75 x 10 - 4
[Ca2+] = 6.25 x 10 - 4
[F-] = 2.5 x 10 - 4
alkalinity = [HCO3-] = 1x 10-3
7. All cause increase in alkalinity.
10. The reaction between the NTA and CaCO3 is
HT2- + CaCO3 ® CaT- + HCO3-
Write the reaction for Ksp of CaCO3,
CaCO3 ® Ca2+ + CO32-
add to it the reaction Ca2+ + HT2- ® CaT-
+ H+ (K')
then add the reversed reaction for ionization of HCO3-
H+ + CO32- ® HCO3-
(1/Ka2 )
This gives the desired reaction. When you add reactions multiply their Ks together, when you reverse a reaction, its K is inverted. So the above reaction has a K expression of:
K = ( Ksp K' ) / Ka2
All these constants are in the chapter. Solving gives K = .739
With this large a K the reaction can be considered to go pretty much to completion. So [CaT-] = [HCO3-] = 1.00 x 10 -3 . Substituting this into the K expression, HT2- is found to be 1.35 x 10 -6
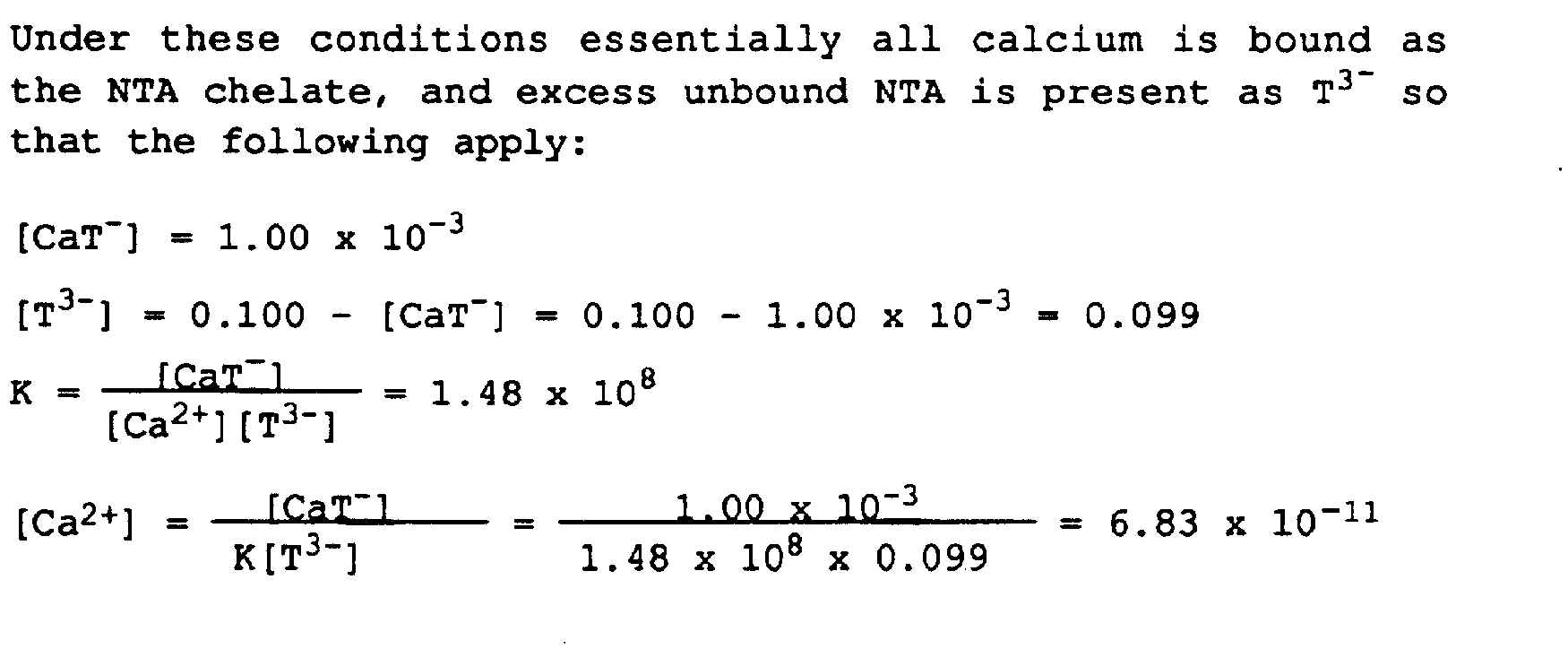
12. 
13. When an oxide layer dissolves slightly, a chelating agent will tie up the metal ions, leading to further dissolution. It is a case of shifting the equilibrium by removing a product. (LeChatelier's principle)
16.