1. Sediment at pH 10, HCl used to displace cations.
150 mmol Na+
5 mmol K+
20 mmol Mg++ (x2 meq/mmol)
75 mmol Ca++ (x2 meq/mmol)
Add these up to get total of 345 meq/100 g sediment. There are no sites occupied by H+, because the sediment is basic.
2. [X] = kPx so [O2] = kO2 PO2 = 1.28 x 10-3 ( 0.5 atm)
To be more accurate, there is a vapor pressure of water included in the 1 atm total pressure. This, at 25oC is 0.031 atm. So PO2 is (1atm - 0.031) x 0.5 atm
[O2] = 1.28 x 10-3 ( 0.484 atm) = 6.2 x 10-4 mol/l
3. Soluble Fe3+ is least likely to occur in natural water--Fe2O3 is very insoluble and will likely precipitate.
4. These precipitates are usually negatively charged and sorb cations by electrostatic attraction. Divalent ions are strongly attracted and removed from solution.
6. Use the Clausius-Clapeyron equation (5.3.20)
Where C1 = 14.74 mg/L, at 273K and C2 = 7.03 mg/L at 308K. Solve for the constant term (deltaH /2.303R) which is -773k. Then use T2 = 323K, T1 = 273, and 14.74 for C1, and the constant as calculated above. Solve for C2, the solubility at 373K (or 50oC). C2 = 5.38 mg/L.
11. Point at which the number of positive and negative sites on the surface of the colloid are equal. There are still positive and negative sites, but the overall charge is neutral.
12. 2 {CH2O} = CH4 + CO2 A mmol of gas at 0 oC and 1 atm occupies 22.4 ml (from ideal gas law).
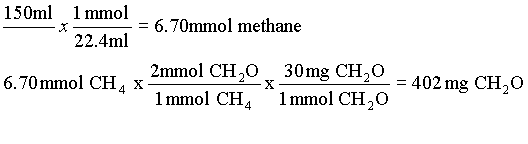
18. A polymer with negatively charged groups bridges colloidal particles, binding them together and acting as a flocculating agent.