

The Earth's Atmosphere
See the Chapman Cycle
Human Activity and the Atrmosphere
In the past two decades, however, scientists have found evidence that human activities are disrupting the ozone balance. Human production of chlorine-containing chemicals such as chlorofluorocarbons (CFCs) has added an additional force that destroys ozone. (CFCs are compounds composed of carbon atoms bonded to chlorine, fluorine.) As was seen earlier, CFCs are stable and thus do not react easily with other chemicals in the lower atmosphere. One of the few forces that can break apart CFC molecules is ultraviolet radiation in a process called photochemical decomposition. In the lower atmosphere, however, CFCs are protected from this radiation by the ozone layer. So, CFC molecules can migrate intact into the stratosphere where they then are photodecomposed. At first, scientists thought CFCs were too heavy to make their way into the upper atmosphere. Although the CFC molecules are heavier than air, the mixing processes of the atmosphere lift them into the stratosphere. The mixing process takes many years, up to fifty, and so the problem is not easily noticed. The ozone in the statophere today is being destroyed by CFCs released many years ago.
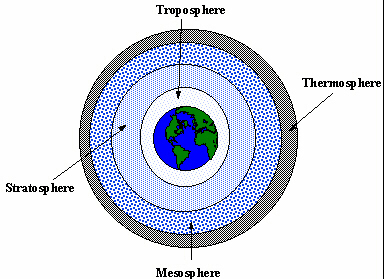
Before discussing the CFC problem, it is necessary to understand how the Earth's atmosphere is broken into layers. There are four distinct areas of air surrounding the Earth. Each has distinctive characteristics which change as the distance from the Earth's surface increases. The area humans live in is known as the troposphere. It begins at the surface of the earth and extends for 7-10 miles (11-16 km). The temperature and pressure decrease rapidly with altitude until the tropopause is reached. The tropopause is an area at which a temperature inversion occurs. This area acts as a nearly impervious barrier to most of the water trying to rise out of the atmosphere. This is important because what little water passes this point can escapes into space.
Above the troposphere is the stratosphere which extends to a point approximately 31 miles (50 km) above the Earth's surface. Temperature remains fairly constant near the tropopause but begins to increase near the upper bound of the stratosphere as solar radiation increases. The water that passes the tropopause forms clouds here. The most important molecule in the stratosphere is ozone.
Next comes the mesosphere. It extends from the top of the stratosphere to an altitude of 50 miles (80 km). Finally, the top of the atmosphere is known as the thermosphere. It begins at 50 miles and continues to outer space. The temperature continues to increase through the mesosphere and the thermosphere.
Ozone is of concern to us when it is in the lower two levels of the atmosphere, the troposphere and the stratosphere. No matter where it is found, ozone is a relatively unstable molecule. An ozone molecule consists of three atoms of oxygen bound together in a triangular fashion. Although it represents only a tiny fraction of the atmosphere, ozone is crucial for life on Earth.
TDepending on where ozone is located, it can either protect or harm life on Earth. In the stratosphere, ozone acts as a shield to protect Earth's surface from the sun's harmful ultraviolet radiation. Without this shield, ultraviolet levels at the Earth's surface would be higher and humans would be more susceptible to skin cancer, cataracts, and impaired immune systems. In the troposphere, however, this same ozone molecule is a harmful pollutant that causes damage to lung tissue and to plants.
The amounts of helpful and harmful ozone in the atmosphere depend on a balance between processes that create it and those that destroy it. An upset in the ozone balance can have serious consequences for life on Earth. Scientists are finding evidence that changes are occurring in ozone levels. The harmful ozone is increasing in the air we breathe, while the helpful ozone is decreasing in our protective ozone shield. In the next few pages, the processes that create and destroy the helpful ozone will be described. Also, the way that humans effect these processes will be discussed.
At the top of the stratosphere ozone is created and destroyed primarily by ultraviolet radiation. The air in the stratosphere is bombarded continuously with radiation from the sun. The ultraviolet rays, which are part of this light, strike molecules of ordinary oxygen (O2) causing them to split into two single oxygen atoms, known as atomic oxygen or oxygen radicals. A freed oxygen atom then can collide with an oxygen molecule (O2), and form a molecule of ozone (O3).
This process absorbs much of the ultraviolet radiation which would otherwise reach the Earth's surface. Ironically, this same ultraviolet radiation also causes the destruction of ozone. When an ozone molecule (O3) absorbs even low energy ultraviolet radiation, it splits into an ordinary oxygen molecule (O2) and a free oxygen atom (O). The free oxygen atom then may bond with an oxygen molecule to make another ozone molecule, or it may steal an oxygen atom from an ozone molecule to make two ordinary oxygen molecules. Some scientists call these processes of ozone production and destruction, initiated by ultraviolet radiation, the "Chapman Cycle."
Natural forces other than the Chapman Reactions also affect the concentration of ozone in the stratosphere. Since ozone is such a highly unstable molecule, it reacts very easily, readily donating an oxygen molecule to nitrogen, hydrogen, or chlorine found in natural compounds. These elements always have existed in the stratosphere, released from sources such as soil, water vapor, and the oceans.
In addition, ozone levels can change periodically as part of regular natural cycles such as the changing seasons, sun cycles and winds. Moreover, volcanic eruptions may inject materials into the stratosphere that can destroy ozone.
Over the Earth's lifetime, natural processes have regulated the balance of ozone in the stratosphere. An easy way to think about the ozone balance is to imagine a plastic bag being filled with water. As the bag fills, a hole is punched in it to allow water to escape. As long as water escapes at the same rate that water is being poured in, the amount of water in the bag will remain the same. Likewise, as long as ozone is being created and destroyed at the same rate, the total amount of ozone will remain the same.
Back To The Overview
 Goto anywhere in the case study
Goto anywhere in the case study