Stratospheric Ozone Layer
Depletion
Page 5
Characteristics
of Freons
In
general, all Freons are carbon compounds containing chlorine, fluorine, and/or
bromine. The most common Freon compounds are chlorofluorocarbons or CFCs in
particular, CFC-12 (See CFC-12)
whose chemical formula is CCl2F2. Freons are used so
widely by industry because of their high densities, low boiling points, low
viscosity, and low surface tension. In addition, they are easily liquefied
making them ideal for use as refrigerants and solvents. All of these properties
made Freons a best seller amongst many industries. Also, the properties made
them useful in many other areas. Freons are widely used as solvents,
propellants, fire extinguishers, and blowing agents.
Before
looking any further into the spread of CFCs, it is important to understand why
they are so chemically useful. Since CFC-12 is the most widespread, it will
serve as a good example of the chemical usefulness of the entire class of
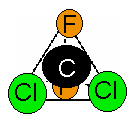
chemicals, known as CFCs. From the discussion above, the molecular formula for
CFC-12 is CF2Cl2. The name of this compound is dichlorodifluoromethane. To
imagine what this would look like in three-dimensional space, begin by
envisioning a pyramid with a triangle as its base. Place the carbon atom in the
center of the triangle with the chlorines and fluorines at the four points.
This is known as a tetrahedral configuration.
Each
halogen is fighting to draw one electron away from the central carbon atom. At
the same time, the carbon holds the electrons in the covalent bonds so it does
not lose them. Since fluorine and chlorine are aggressive elements, the bonds
that are formed are very strong. The chlorine acts as a stabilizing agent
giving even more stability to this molecule.
These
strong covalent bonds and added chlorine stability make CFC-12 inert. This
means it does not react with other molecules in its surroundings. Besides its
chemical inertness, CFC-12 has many other chemical and physical properties that
make it ideal for use in many industrial fields. Its boiling point allowed it
to be used as a refrigerant eliminating the danger of explosion or toxicity
that was associated with ammonia. Also, because it was inert and nontoxic, it
could be used to blow foam for formation of containers or insulation. These
same properties made it perfect in medical inhalers and aerosol spray cans.
Other applications also arose as the other CFCs were produced. The low costs of
production coupled with their versatility and widespread appeal helped them to
find their way into many industrial operations.
The same
property of inertness that makes CFCs so useful in industry would one day prove
to be what makes them so dangerous to the planet. Even as CFCs became more
widely spread in industry, they were slowly being vented to the atmosphere. At
the time this was not seen as bad practice because they were thought to be
safe. Unfortunately, CFCs do not naturally biodegrade. As a result, they
persist in the atmosphere. Through natural processes, they make their way up
into the stratosphere where the real problem begins. From their inception until
the mid seventies, however, CFCs where seen as safe, useful, and
non-controversial.
Next Page
 Goto
anywhere in the case study
Goto
anywhere in the case study


