MEMBRANE RESEARCH PROJECTS
Membranes have become a viable alternative to conventional sample preparation techniques such as liquid-liquid extraction (LLE), solid-phase extraction (SPE), rotary evaporation and solid phase microextraction (SPME). They have been applied to a wide range of compounds. Below are a few examples of the versatility of membrane extraction techniques.
1. Continuous On-line monitoring of haloacetic acids
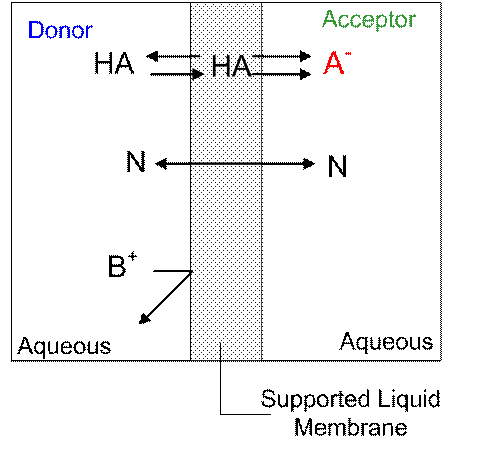
Disinfections by-products (DBPs) refer to those compounds formed upon the addition of disinfecting agents such as chlorine, chlorine dioxide and chloramines for the purpose of water treatment. One such group of DBPs are haloacetic acids(HAAs). Many of these compounds have been determined to be hazardous to human health and as such are monitored by the EPA. The EPA methods (EPA method 552.1, 552.2 and 6251)methods involve cumbersome liquid–liquid extraction or ion exchange and derivatization, followed by GC-ECD detection and therefore is time consuming, consume large amounts of solvent and not amenable to automation. We have recently developed an automated on-line system capable of continuous real- time monitoring as well as a microfluidic system. An automated on-line system allows us to get real-time data and reduces sample handling and therefore is less prone to errors. Microfluidic devices shown in Fig. 1 have large surface area to volume ratios, consume small amount of reagents and can be inexpensively produced. With these systems, the detection of all nine HAAs at ppb levels was possible. Both techniques are based on supported liquid membrane extraction (SLME) which is essentially a three-phased system where an organic phase is sandwiched by two aqueous phases. The analyte (A-) moves across a pH gradient and are trapped in the acceptor phase as shown below.

Fig. 1 Schemmatic representation of SLME of acids
2. Barrier-film microscale membrane extraction
Membrane extraction typically involves the use of an organic liquid immobilized within the pores of a hydrophobic porous membrane. The extraction is dependent upon this liquid remaining in the membrane pores throughout the procedure. This liquid may be lost via diffusion or dissolution in the acceptor solution. Loss of the extractant results in analyte loss and consequently low enrichment factors (EF). To enhance membrane stability we have used a dip-coating procedure to coat the membrane in a liquid immiscible with the acceptor. This liquid is referred to as the barrier film. This is shown below. This facilitates more rigorous extractions, decreases extractant loss and results in higher EFs. This technique has been successfully used to detect polyaromatic hydrocarbons such as anthracene, fluorene and phenanthrene in water at parts per trillion levels as well as carbamate pesticides such as carbaryl, carbofuran and methiocarb.
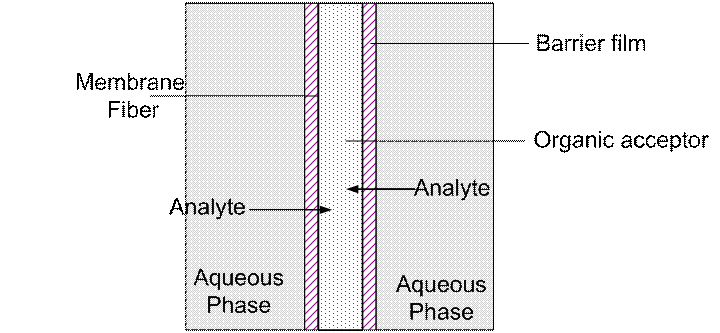
Fig. 2 Schemmatic diagram of barrier-film enhanced SLME. The barrier film coats the membrane and so stabilizes the organic extractant and facilitates greater enrichment of the analytes in the membrane lumen.
3. Pervaporative concentration of pharmaceuticals
Whereas the other techniques were geared towards semi-volatile organic compounds (SVOCs), pervaporation is suitable for volatile organics and in this case refers to the permeation of volatile organics across a membrane barrier into a gas phase. Continuous on-line analysis has been essentially non-existent in pharmaceutical manufacturing. Typically, samples are collected at various steps in the process, and sent to the laboratory for analysis. The samples undergo various sample preparation steps, such as, extraction and concentration prior to detection. These steps are both labor and time intensive. Evaporative techniques are usually used for analyte concentration. Essentially, it concentrates the sample by selectively removing the solvent. Most classical evaporative techniques are relatively laborious procedures involving multiple handling steps which tend to results in increased error. We have been able to develop on-line membrane preconcentration for monitoring pharmaceuticals at trace levels. Using polar solvent permeable Nafion membranes, solvent was reduced by more than 90% allowing the concentration of compounds such as 1,2-diphenylhydrazine and naphthylacetonitrile shown in Fig. 3
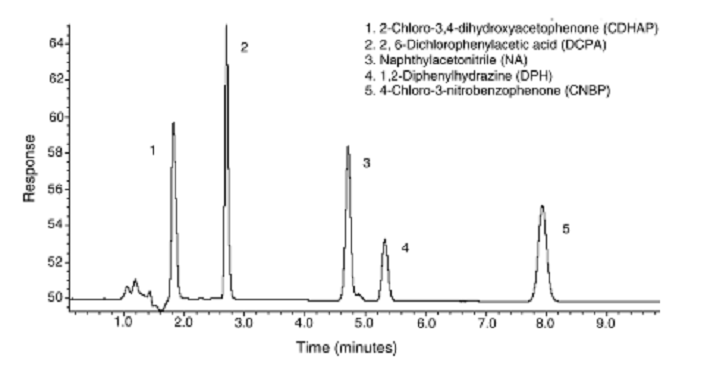
Fig. 3 HPLC separation for 5 pharmaceuticals after membrane pervaporation