Green Chemistry in Teaching Laboratory
Microwave Induced Reactions
|
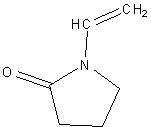
Synthesis of Polyvinylpyrrolidone (PVP) PVP is a water soluble polymer that also dissolves in other polar solvents. At dry conditions, it is a white blistering powder that absorbs moisture. From a solution it readily forms films which have been employed for coating purposes. PVP is used as binders for the formulation of pharmaceutical tablets, for moistening various personal care products, as food additives, and adhesives, etc. This has also been employed as an excellent blocking agent in Southern blot analysis (molecular biology technique used for detection of specific DNA sequence in DNA samples). PVP is synthesized via a free radical polymerization reaction starting from the vinylpyrrolidone (VP) monomer, using a free radical initiator such as Azobisisobutyronitrile (AIBN).
Scheme 1: Reaction for the synthesis of polyvinylpyrrolidone Materials and Instrumentation
VP and AIBN were purchased from Sigma Aldrich. In comparing the reaction efficiency, these experiments are carried out in a microwave oven and also in a hotplate. Experimental Procedures Two bottles (20 ml) containing six to eight mg of free radical initiator, 2,2’-Azobisisobutyronitrile (AIBN), 2 ml vinylpirrolidone (VP) and 2 ml water were stirred for about 20 min at room temperature to dissolve all the AIBN into water. One sample was placed in the microwave oven and the reaction was carried out for 3 minutes and the polymer was formed. The other sample was placed in a hot plate at its maximum power. The polymer was formed in 13 min. The pictures of starting materials and products are presented in Figure 3. The results are presented in the Table below. The net saving in energy was quite evident.
VP
(starting materials)
Figure 5. Photographs of initial reactants and final product. Result &Discussion: The formation of white jelly-like solids indicated the completion of the polymerization reaction. Once it is allowed to dry, it forms white flakes which readily absorb atmospheric moisture. Table 2. The energy consumed for the PVP synthesis by different heating methods
Therefore, the percentage of energy saved by the microwave oven over the conventional oven is,
|