|
|
Green Chemistry in Teaching Laboratory Microwave Induced Reactions |
||||||||||||||||||||||||||||||
|
|
|
||||||||||||||||||||||||||||||
Green Chemistry in Teaching Laboratory
Microwave Induced Reactions
Saponification of Fat - Synthesis of Soap
Saponification is the process of making soap from alkali and fat (or oil). Vegetable oils and animal fats are fatty esters in the form of triglycerides. The alkali breaks the ester bond and releases the fatty acid salt and glycerol. If necessary, soaps may be precipitated by salting out with saturated sodium chloride. Usually, sodium hydroxide is used in formation of hard soap while potassium hydroxide is used in case of soft soap.
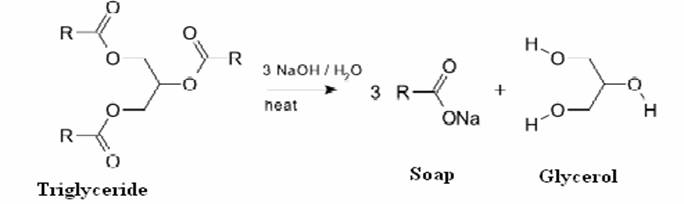
Scheme 2. Saponification Reaction.
Mechanism of cleaning by Soap
It is not possible to remove dirt (especially oil and grease) by just using water. The non-polar components present in the dirt repel the polar constituent of the solvent. In presence of a detergent (soap) which has both polar and non-polar ends, the non-polar ends of the detergent which is repelled by water interacts with the nonpolar grease. At the same time, the polar ends are attracted towards the hydrophilic molecules. Thus the two complementary polar and nonpolar components of the dirt are dissolved and removed during washing. The soap forms micelles in water, where the polar ends align along the circumference and non-polar constituents carrying the nonpolar species are remain in the center of the micelle.
* Figure 8. Typical micelle formation
*Source: http://www.images.google.com
Materials Required
Animal Fat
Ethanol
6N Sodium Hydroxide
Sodium Chloride
Isoproply alcohol
Two 100 ml Beaker
Two glass rods
Two 250ml conical flask
Distilled water
Microwave
Hot plate
Safety goggles
Towels for clean up
Stop watch
Experimental Procedure
Ten grams of commercially available animal fat/shortening was weighed and dissolved in 50 ml of ethanol by constant stirring. To this mixture was added 15ml of freshly prepared 6N sodium hydroxide solution. This mixture was heated on a hot plate until all the fat was completely dissolved. 20ml of distilled water was added and the mixture was cooled on an ice bath. The cooled mixture was then poured into a beaker containing 50ml of 0.2 % Sodium Chloride solution. Soap was formed upon cooling. The solution was filtered to separate the soap from the glycerol thus formed. The experiment was repeated using microwave. The corresponding efficiencies and energy consumptions were calculated.
Result & Discussion
As a result of the Saponification process, the fatty acids are hydrolyzed in presence of an alkali so as to form salts of alkali and alcohol. Upon cooling of the dissolved mixture, solid soap was observed the end of the process. Energy consumed by each process is tabulated below.
One way to test the formation of soap is by dissolving the solid in water and checking foam formation. It can also be confirmed by performing pH test, which involves dissolving the soap in a freshly prepared 1:3 water and isopropyl alcohol mixture. The dissolved soap solution can be tested with phenolphthalein indicator and the soap formation is indicated by the color of the solution. A dark pink and clear solution indicates presence of excessive caustic solution, the one with colorless or yellowish clear solution indicates a fairly neutral pH, hazy solution indicates untreated oil, hazy and pink solution indicates the reaction was complete. A clear, pale pink solution indicates good results.


(a) (b) (c)
Figure 9. (a) Untreated soap solution, (b) Hazy pink solution with untreated oil, (c) Clear pink
solution indicating the formation of soap.
Table 4. The energy consumed for saponification of 10g of animal fat.
Heating Device
Time (min)
Power Rating (KJ/min)
Actual Energy Consumed (KJ)
Microwave Oven
1
51.0
36
Hot Plate
4
51.9
108
Therefore, the percentage of energy saved by the microwave oven over the conventional oven as recorded by the power meter is,
The Saponification process using conventional heating took four times as much time to complete than the microwave process, and consumed more energy. The quality of soap was also tested. From the Fig. 9 (c) we see the solution thus formed after the addition of phenolphthalein, was a clear pink solution indicating the formation of good soap with a nominal pH in the range of 7-9. In Fig 9 (b) we see a hazy pink solution with yellow droplets in it, indicating the presence of unreacted oil. The solution also appears dark in color indicating the presence of excessive caustic soda. Fig 9 (a) shows a clear solution which indicates the absence of soap formation.
The amount of energy consumed by the hotplate was significantly higher than what was consumed by microwave. This was attributed to the direct interaction of the reactants with the microwave radiation.