Green Chemistry in Teaching Laboratory
Microwave Induced Reactions
|
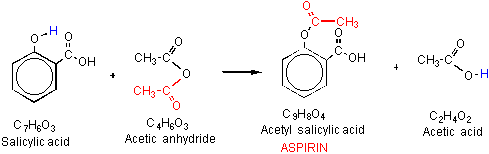
Synthesis of Aspirin
Aspirin or acetyl salicylic acid (ASA) is a derivative of salicylic acid, which is used as a pain reliever for various body ailments such as head ache. Aspirin shows anti-platelet or anti- coagulant properties by inhibiting the prostaglandins, thereby repairing damaged blood vessels. Hence aspirin is used in low doses on long term basis in treatment of heart attacks, strokes and in people having high risks for formation of blood clots. The synthesis of acetyl salicylic anhydride from salicylic acid and acetic anhydride is catalyzed by phosphoric acid. The efficiency is estimated by the time taken to complete the reaction.
Scheme 3. Reaction equation for the synthesis of Aspirin
Materials Required
Experimental Procedure 18 ml of acetic anhydride was slowly added to 10 grams of salicylic acid in a 250 ml Erlenmeyer flask in the hood. 10 to 20 drops of 85 % phosphoric acid was carefully added to the solution and mixed thoroughly. The mixture was heated on a hot plate until all the salicylic acid was dissolved. Once the reaction was complete, 20 drops of distilled water was cautiously added to the mixture followed by 20 ml of distilled water. The solution was cooled on an ice bath until aspirin crystallized. In event of no crystal formation, the walls of the flask were scratched with a stirring rod to initiate crystallization. The crystals were filterer using a Buchner filter and extracted using chilled water. The solid was then dried in an oven at 100 °C for about 30 minutes, weighed and the yield was calculated. The experiment was repeated in a microwave oven and the yield was compared. Further analysis could include melting point, IR and NMR measurements.
Figure 10. Represents the final product obtained after synthesis. Results and Discussion Solid salicylic acid structures were dissolved in presence of acetic anhydride which is catalyzed by acid. The dissolved mixture on cooling produced white crystals of Aspirin. The corresponding energy consumed by each method of heating is tabulated below. Table 5. The energy consumed for the synthesis reaction.
From the data presented in table 5, the microwave method of synthesis proved to be more efficient than the hotplate. Fig. 10 shows the final aspirin product obtained after synthesis process. The synthesis process was enhanced in the case of microwave method of heating thereby saving energy. The microwave process also yielded a slightly higher amount of product than the hot plate. The percentage of energy saved by the microwave oven over the conventional oven as recorded by the power meter,
|