Green Chemistry in Teaching Laboratory
Microwave Induced Reactions
|
De-emulsification of Oil by heat
De-emulsification is one other practical application of heat where mixtures of two different immiscible liquids are separated. Emulsion is a mixture of two different immiscible liquids, where in each liquid is made miscible by addition of emulsifying agent or a surfactant. In this experiment, oil and water is taken as two immiscible liquids which are mixed in different proportions and mixed with a surfactant (generally detergent). Separation of this emulsion is achieved by application of heat. Addition of surfactant causes a micelle formation by hydrophilic and hydrophobic interactions due to the polar molecules present in the mixture. Application of heat causes breakdown of these micelles, interrupting the hydrophilic-hydrophobic interactions. This leads to the separation of the two immiscible liquids. The process of de-emulsification is applied in purification of heavy crude oil in petroleum & paint industries.
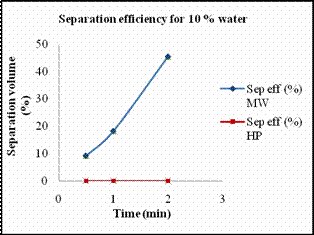
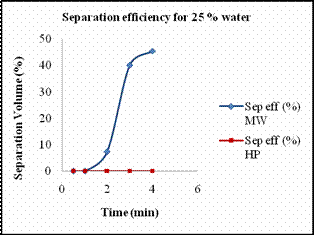
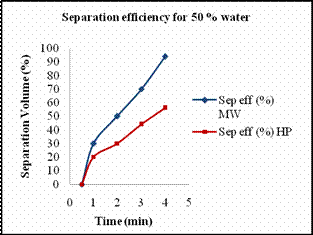
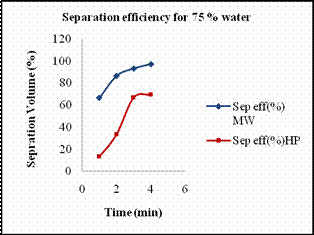
Scheme 5. 2 De-emulsification reactions Materials Required · Two 1000ml graduated glass beaker. · Two glass rods for stirring. · Measuring jar. · Cooking oil. · Distilled water. · Sodium dodecyl sulfate (SDS). · Stirring Machine. · Safety goggles. · Towels for clean up. · Stop watch. Experimental Procedure Previously we reported the temperature profile of de-emulsification process of oil and water emulsion. The emulsion mixtures prepared were not stable enough. Hence we prepared stable emulsions with SDS as emulsifying agent. Emulsion solutions containing 110ml of different concentrations of 10%, 25% and 50% solutions of water in oil with SDS as emulsifying agent. The amount of SDS added was proportional to the percentage of water added. In case of 10 % water, the amount of SDS used was about 0.05 g while in case of 25 % water, 0.1g of SDS was used and in that of 50 % water, 0.2g of SDS was used. The solutions were heated at every interval time in order to determine the amount of water separated. Heating the solution every minute the volume of water separated was recorded. Results and Discussion Figures 11 (a) and 11(b) show the amount of water separated by hotplate in case of 10 % and 25 % in comparison with microwave. At lower percentage of water, hot plate hardly separated the water from the mixtures. As it required more amount of energy to break down the micelle bonds. In case of microwave radiation, the direct heat applied by it was sufficient enough to break down the strong micelle bonds formed at lower percentage of water content. Higher the percentage of water, more unstable the micelle bond becomes, hence it requires less energy to break down the micelles in order to separate the emulsion. Thus from the Fig 11 (c) we see that for a 50 % micelle mixture a substantial amount of water separated by hot plate heating too. But for the same period of time the amount of water separated by microwave was comparatively higher than that of conventional heating. The amount of water separated by both methods of heating for separation of 50 % oil in water mixture, and their respective energy consumption is tabulated below.
(a) (b)
(c) (d) Figure 13. (a) Volume of water separated versus time for 10 % emulsion mixture, (b) Volume of water separated versus time for 25 % emulsion mixture, (c) Volume of water separated versus time for 50 % emulsion mixture. (d) Volume of water separated from 75 % emulsion mixture. The amount of water separated by both methods of heating increased with increase in amount of water content. Table 7. The energy consumed and the concentration of iron extracted by different heating methods.
Difference in Calculated and Recorded Values: Energy = Power x Time Microwave Oven: Energy Consumed = 51.0 KJ/min x 3 min = 153 KJ Hot Plate: Energy Consumed = 51.9 KJ/min x 3 min = 155.7 KJ The percentage of error for microwave oven is,
and the percentage of error for hot plate is,
|